Probing ion channels
High-Speed AFM for elucidating the dynamics of biological membranes
“My first experience of using atomic force microscopy (AFM) was during my final year as an undergraduate at Nagoya Institute of Technology,” says Ayumi Sumino, an assistant professor, at the Nano Life Science Institute (WPI-NanoLSI). “My project was related to fabricating microbial fuel cells using photosynthetic membrane proteins. The ability to image biological specimens on the nanometer scale stimulated my curiosity in both nanoimaging and its applications for elucidating molecular mechanisms of biological membranes.”
Sumino’s interest in nanoimaging of biological specimens led her to enroll as a postdoc and JST/PRESTO researcher at the Fukui University’s Faculty of Medical Sciences where she used AFM technology to study the gating and cooperative clustering of ion channels in lipid membranes.
“Lipids and proteins self-assemble to form fluidic sheets with thicknesses of only about 5-10 nm,” explains Sumino. “We only have limited knowledge about their molecular mechanisms. Such insights would contribute to understanding life and developing medicines. I have been at the NanoLSI for three years and the focus of my research is on understanding the structural dynamics and molecular mechanisms of biological membranes including regulatory mechanisms of ion channels. The rich history and know-how and wide range of AFM systems at the NanoLSI are vital for achieving the aims of my research; I am impressed with the research environment and support here. I am expecting our first child later this year and hope that I can balance research with family life. I have noticed that only the female washrooms have facilities for changing diapers. If the men’s rooms had similar amenities then my husband, who is also a researcher at Kanazawa University, would be able to share the load of changing diapers during the day!”
High speed atomic force microscope for imaging ion channels
Sumino and her colleagues at the NanoLSI are focused on four aspects of the dynamics of biological membranes: structural changes of membrane proteins; association and dissociation; clustering and dispersion; and morphological change such as pore formation and bending. Sumino is using the state-of-the-art AFM instruments developed by researchers at Kanazawa University that include the high speed atomic force microscope (HS-AFM). This system has a spatial resolution X/Y~1 nm ; Z~0.1 nm and produces successive images at ~80-ms intervals thereby enabling the visualization of nanostructure and dynamics of membranes and proteins in aqueous solutions. “In 2014 we published a paper on the AFM imaging of clustering and diffusion of ion channels,” says Sumino. “This paper demonstrated the power and potential of AFM for imaging ion channels in wet samples.”
Ionic channels in biological membranes act as gatekeepers or ‘switches’ controlling the movement of ions including sodium, potassium, and calcium, between the extracellular and intracellular regions of cells. “Incorrect permeation of ions through channels can lead to dysregulation of the membrane potential, resulting in critical dysfunction of cells and related aliments including irregular heartbeat, kidney disorders, and periodic paralysis,” explains Sumino. “So it is vital to understand the dynamics of ion channels.”
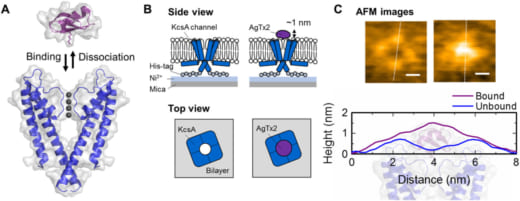
Recent findings include experimental observation of the underlying binding dynamics that govern the blocking of potassium channels by the scorpion toxin Agitoxin-2 (AgTx2)—a well known toxin that blocks potassium-ion permeation by binding onto extracellular surface of the potassium channel by electrostatic and hydrophobic interactions. “The aim of these experiments was to determine the precise binding model responsible for the AgTx2 onto the KcsA potassium channel,” says Sumino. “We used HS-AFM to capture AgTx2 binding at 100ms/frame and extracted binding dynamics of the AgTx2 as changes in the height by about 0.5 to 1.0 nm. The results were published in Science Advances.”
There are three well known binding models for ligand-receptor: the lock and key model; induced fit model; and the conformational selection model. “The results of these experiments led us to propose a new model, called the ‘four state model’ to explain the observations,” explains Sumino. “The four-state model including high- and low-affinity binding states fitted our experimental data well. The AgTx2 effectively blocks potassium ion channels by using induced-fit pathway. I want to continue to clarify other phenomena related biological membranes.”
References
- Sumino, T. Sumikama, T. Uchihashi, and S. Oiki, “High-speed AFM reveals accelerated binding of agitoxin-2 to a K⁺ channel by induced fit”, Science Advances, 5, eaax0495, (2019).
https://doi.org/10.1126/sciadv.aax0495
- Sumino, T. Uchihashi, S. Oiki, “Oriented Reconstitution of the Full-Length KcsA Potassium Channel in a Lipid Bilayer for AFM Imaging”, J. Phys. Chem. Lett. 8, 785-793, (2017).
https://doi.org/10.1021/acs.jpclett.6b03058
- Sumino et al. “Gating-Associated Clustering–Dispersion Dynamics of the KcsA Potassium Channel in a Lipid Membrane”, J. Phys. Chem. Lett. 2014, 5, 3, 578-584, (2014).
https://doi.org/10.1021/jz402491t
Further information
Ayumi Sumino, NanoLSI
https://researchmap.jp/suminof?lang=en