Hematopoietic stem cells and leukemia
Imaging the interaction between Forkhead box proteins and DNA by atomic force microscopy

Sha Si, Assistant Professor
“I joined the NanoLSI in May 2018 and am grateful for the opportunity to conduct research on cancer cells with some of the world’s leading experts in the field,” says Sha Si, an assistant professor, at the WPI Nano Life Science Institute’s Cancer Research Institute. “The research environment at the NanoLSI offers access to world class equipment, interaction with scientists from different fields for immediate and in-depth collaboration, and full support from the administration for research issues and for living in Kanazawa. Also, I and my other female colleagues feel very comfortable working here.”
Si has a unique set of academic qualifications that are proving to be significant assets for her research at the NanoLSI. She completed a five year medical degree at the Changzhi Medical College, Shanxi Province, China. Next, Si decided to pursue her interest in Japanese culture and after a year at a Japanese language school in Tokyo, was accepted as a researcher at the Department of Cellular and Molecular Medicine before starting her doctorate. “My degree in medicine and graduate school studies in cellular and molecular medicine are proving to be very useful for my research at the NanoLSI.”
Mechanisms for stem cell maintenance: Hematopoietic stem cells and leukemia
Stem cells produce new cells for the body to replace old or damages ones, and these cells have the potential to develop into any type of blood cell as they divide and mature via a process known as differentiation. And, stem cells also have the capacity of unlimited self-renewal to produce new stem cells. Notably, blood stem cells can go out of control and become cancer stem cells that give rise to blood cancers, including leukemia, which is an aggressive blood cancer characterized by differentiation blockade and abnormal cell proliferation.
Specifically, acute myeloid leukemia (AML) is a form of leukemia and the survival rate is only 25% over five years. Research shows that the transcription factor Forkhead box O (FOXO) family block differentiation of leukemia. Si and her colleagues confirmed that FOXO inhibitor can induce leukemia cell differentiation. They successfully identified FOXO-downstream molecules essential for leukemia differentiation blockade. Furthermore, they identified FOXO-response element (FRE) as a critical cis-regulatory element for leukemia differentiation blockade. All of these suggest a possible therapeutic approach to AML by inhibition of FRE activity.
Observation of FOXO protein and DNA by AFM
“I am devising methods to observe the interaction between FOXO and DNA using AFM,” says Si. “The ultimate goal is the development of new drugs to induce leukemia cell differentiation. My approach is to characterize the structure of FOXO in different forms using AFM.”
It has not been clear how FOXO protein interacts genomic DNA, because the information of structure of FOXO family is limited. Since the FOXO family are disorder proteins, it is technically difficult to analyze crystal structure of whole protein. However, it is possible to characterize dynamics of the FOXO protein by AFM. Therefore, Si believes that she will have a chance to obtain valuable information about dynamics of FOXO protein, which lead to deep understanding how transcription factors interact target DNA and how inhibitors affect behavior of FOXO proteins.
Si emphasizes that these experiments give deep insights into how FOXO regulates target genes and how drugs affect FOXO functions. Next, Si will focus on understanding the molecular mechanisms underlying the development of acute myeloid leukemia and study the roles of abnormal diets in the normal hematopoiesis and leukemogenesis.
“I hope that my research can contribute to the treatment of leukemia on a global scale.”
References
- K. Naka, et al. “TGF-β–FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia”, Nature 463, 676–680 (2010).
https://doi.org/10.1038/nature08734
- Sha Si et al. “Hematopoietic insults damage bone marrow niche by activating p53 in vascular endothelial cells”, Experimental Hematology, 63, 41-51, (2018).
https://doi.org/10.1016/j.exphem.2018.04.006
- Sha Si et al. “Loss of Pcgf5 Affects Global H2A Monoubiquitination but Not the Function of Hematopoietic Stem and Progenitor Cells”, PLoSOne. 11(5):e0154561.
https://doi.org/10.1371/journal.pone.0154561
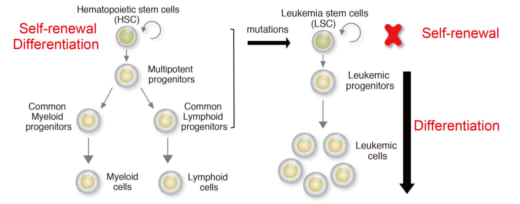
1. The goal of treatment for leukemia is to lose self-renewal property of leukemia stem cells and induce differentiation.
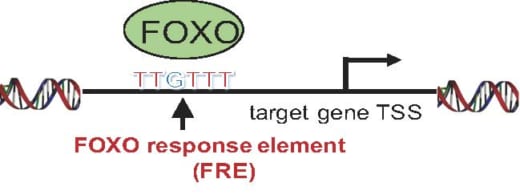
3. FOXO bind to consensus DNA sequence, FOXO response element (FRE).
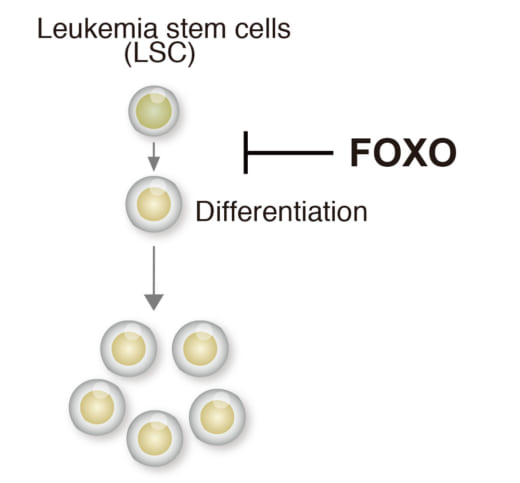
2. Forkhead box O (FOXO) family block differentiation of leukemia cells.
Posted May 18,2020

