Innovative high-speed AFM technology for visualizing protein dynamics
HS-AFM has yielded unprecedented insights into the dynamics of gene editing and living mammalian cells

Mikihiro Shibata, Professor, Nano Life Science Institute (WPI-NanoLSI), Kanazawa University
Four billions years of evolution
“I am fascinated by human development and our existence as a species for four billion years,” says Mikihiro Shibata. “How and why we got here are two fundamental questions. From the perspective of biological processes, our imagination is powered by electrical signals in neurons and synapses. That leads us to think about the dynamics and functions of proteins and their links with diseases. Answering these questions in the motivation for my research.”
High speed atomic force microscopy (HS-AFM)
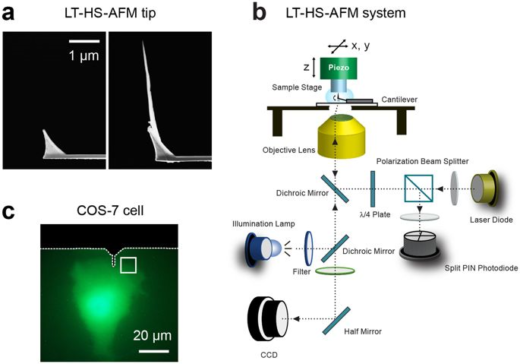
Shibata is using HS–AFM to gain deeper insights into the dynamics of biological processes. He started this research when he joined the lab of Toshio Ando as a JSPS Research fellow (SPD). The HS-AFM technology at Kanazawa University was developed by Professor Ando. It enables high speed and high resolution imaging of one frame in 0.1 s in liquid environments [1, 2]. This performance is possible because the Kanazawa system has a short cantilever of only nine micrometers in length and high speed scanner stage operating at 200 kHz in the vertical direction.
High speed imaging of protein dynamics
In 2010 the Kanazawa group published the results of a major breakthrough in high speed AFM. Shibata showed that illuminating bacteriorhodopsin with light caused the cytoplasmic portions of this so-called light-driven proton pump, to move into contact with adjacent trimers [3]. “This was a major advance in the applications of HS-AFM,” says Shibata. “It gave us the confidence to try even more challenging experiments on visualizing the dynamics of proteins.”
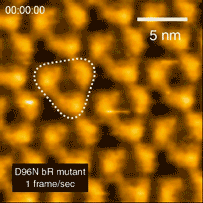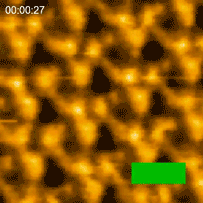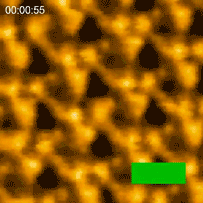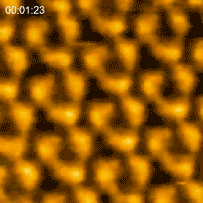
Bacteriorhodopsin
Dynamics of gene editing technology and CRISPR-Cas9
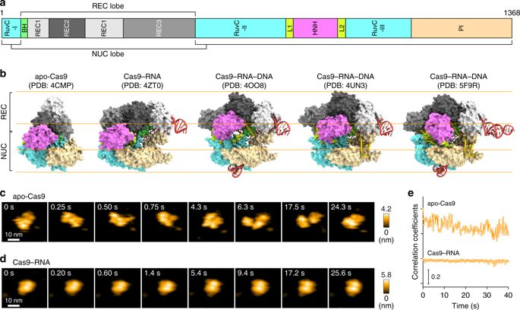
CRISPR-Cas9 technology holds promise as a means of producing precise changes to the genome of living cells. But visualizing the nanoscale process of ‘gene editing’ with CRISPR-Cas9 has been challenging. In 2017 Shibata and his colleagues reported on the real-time visualization of the process of CRISPR-Cas9 cleaving DNA [4].
In related research, Shibata imaged large mammalian cells using very long (approx. 3 micrometers) AFM amorphous carbon tips, thereby overcoming limitations due to collisions between cantilevers and cells [5]
“Recently, we are working on combining optical microscopy with HS-AFM for increasing the functions of our technology,” says Shibata. “Other issues for the future include imaging natural samples, as opposed to highly purified ones that are used the moment. And, identifying proteins is also an important avenue to pursue.”
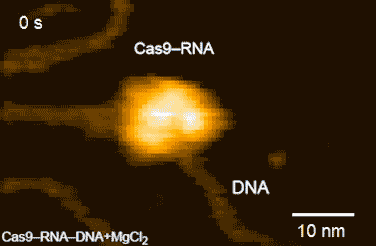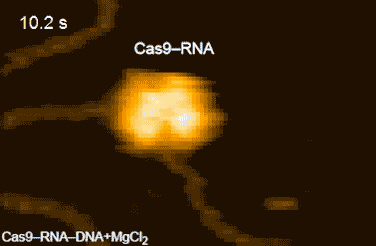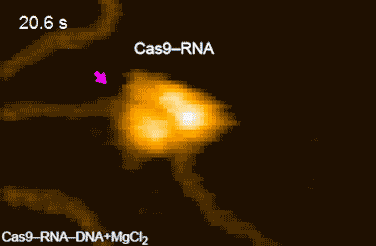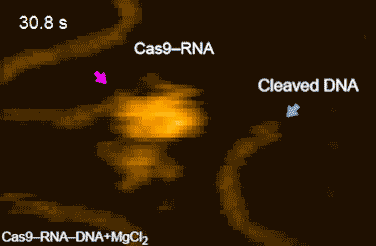
DNA cleavage by CRISPR-Cas9
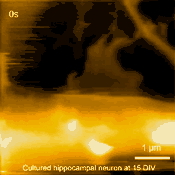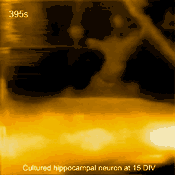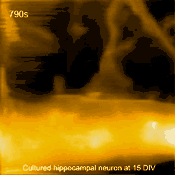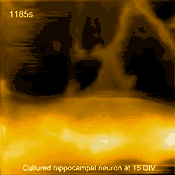
Living cultured hippocampal neuron
Research highlights
Notable publications include, “Real-space and real-time dynamics of CRISPR-Cas9 visualized by high-speed atomic force microscopy”, Nature Communications [4], and “Long-tip high-speed atomic force microscopy for nanometer-scale imaging in live cells”, Scientific Reports [5] .
References
- T. Ando, et al., “A high-speed atomic force microscope for studying biological macromolecules”, PNAS, 98, 12468-12472, (2001); https://doi.org/10.1073/pnas.211400898
- N. Kodera et al. “Video imaging of walking myosin V by high-speed atomic force microscopy”, Nature, 468, 72-76, (2010).
- M. Shibata et al., “High-speed atomic force microscopy shows dynamic molecular processes in photoactivated bacteriorhodopsin”, Nature Nanotechnology 5, 208–212, (2010).
- M. Shibata et al., “Real-space and real-time dynamics of CRISPR-Cas9 visualized by high-speed atomic force microscopy”, Nature Communications, 8, 1430 (2017).
- M. Shibata, et al, “Long-tip high-speed atomic force microscopy for nanometer-scale imaging in live cells”, Scientific Reports, 5, 8724, (2015).