Molecular biology: Making sense of nonsense
Clarifying cellular quality control mechanisms of nonsense-mediated messenger RNA decay and potential therapeutic applications

Hanae Sato, Assoc. Prof., Nano Life Science Institute (WPI-NanoLSI), Kanazawa University
Hanae Sato joined the NanoLSI in September 2022. She specializes in biochemistry, genetics, and live cell imaging. “My overall research spans across ribonucleic acid (RNA) and chromatin biology, that is material complexes of DNA and proteins found in cells of organisms that have clearly defined nuclei – so called eukaryotic cells,” says Sato. “Recently, I am focused on clarifying decay pathways of messenger RNA (mRNA), in a really exciting area known as nonsense-mediated mRNA decay (NMD).”
Nonsense-mediated mRNA decay is a critical ‘quality control pathway’ that detects and eliminates mRNAs containing premature termination codons — sequences of three nucleotide bases in mRNA that trigger signals to stop protein synthesis in cells. In the context of this research, translation refers to the process of translating the nucleotide base sequences of mRNA molecules to sequences of amino acids during the process of protein synthesis.
Harnessing the power of genetics and live cell imaging
“My approach to research is based on integrating techniques that are widely used in biotechnology,” explains Sato. “In particular, I use the Next-Generation Sequencing (NGS) approach and live cell imaging. I can capture single molecules of mRNA in live cells. My methodology enables the detection of motion and localization of mRNA molecules during the process of gene expression including transcription, mRNA export, translation, and decay in living cells.”
Moving to the USA to expand horizons and learn new skills and networking
After completing her doctorate at the University of Tokyo, Sato moved to the USA, where she honed her skills in NMD and chromatin research under the guidance of Drs. Lynne Maquat at the University of Rochester, and Robert Singer and John Greally at the Albert Einstein College of Medicine. “My mentors in the USA have played a central role in enhancing my understanding of the challenges and approaches of NMD and chromatin research,” says Sato. “Having the opportunity to work in the USA enabled me to not only learn more about this area of research but also to form networks with key players within the scientific community; such networks are an important aspect of conducting international research.”
Collaboration at the NanoLSI and potential of nonsense suppression therapeutics
“I think that my single molecule mRNA technology to capture transcription or mRNA translation and mRNA localization is a great candidate for establishing collaborative research at the NanoLSI,” says Sato. “I am looking forward to discussing potential projects with my colleagues here.”
Sato is exploring possibilities of wider ranging applications for her knowledge of mRNA and gene expression processes, in particular expanding her research into RNA therapeutics based on nonsense suppression therapy. Sato explains that nonsense suppression therapy is a strategy for finding solutions to health disorders associated with nonsense mutations. Despite attempts to develop drugs for this strategy, it has not been possible to achieve sufficient drug-induced nonsense suppression due to substantial reductions of nonsense transcripts by NMD.
“I plan to investigate the role of nonsense suppression for NMD inhibition and identify the targets or pathways involved in nonsense suppression for the efficient recovery of full-length functioning proteins,” explains Sato. “I want to develop drug therapies to treat disease-related genes with nonsense mutations for the benefit of society.”
Comparisons of research in the USA and Japan
Sato has experience of working in academia in Japan and USA. She says that one of the main differences is the daily interaction between researchers. “In the US I found much closer communication between principal investigators (PIs) and post-docs or students than in Japan,” recalls Sato. “Students did not hesitate to make their opinions known even if they were contrary to those of PIs. The PIs listened and things moved forward. Such open discussion is one of the main differences between approaches to research in the USA and Japan. In the USA I learned how to find solutions to problems to drive my research forward.” Sato adds that she would like to act as a bridge between cultures to encourage greater interaction with acknowledged international researchers overseas.
Women in science
“In Japan there are very few female researchers, and the government is trying to encourage more women into the sciences,” says Sato. “I may need to take an active role as a female researcher to encourage other women to consider careers in science, especially as independent researchers. I could share my experiences of balancing looking after children with being an independent research scientist. Currently, I am setting up my lab and recruiting people. I am very excited about starting research with my own team and sharing my experience in the USA at the NanoLSI.”
Research highlights: Single cell assays and heritability of chromatin states
Sato has several ongoing projects with recent results including the establishment of a novel single cell assay to assess mRNA decay and NMD efficiency in single cells [1]. “This assay is a unique approach in NMD research,” says Sato. “It enables studies on the intracellular variability of nonsense-mediated mRNA decay within cell populations. This approach enables the investigation of a rare but important cell population that is not detectable with a general biochemical approach.” The results from these studies imply the NMD efficiency is variable and depends on physiological conditions, offering valuable insights into unknown regulatory pathways resulting in the escape of NMD.
Another notable finding published recently is, ‘Retargeting of macroH2A following mitosis to cytogenetic-scale heterochromatic domains’ [2].
References
- H. Sato, R.H Singer, Cellular variability of nonsense-mediated mRNA decay. Nature Communication 12, 7203 (2021).
https://doi.org/10.1038/s41467-021-27423-0 - H. Sato, B. Wu, F. Delahaye, R.H. Singer, J. M. Greally Retargeting of macroH2A following mitosis to cytogenetic-scale heterochromatic domains, J Cell Biol (2019) 218 (6): 1810–1823.
https://doi.org/10.1083/jcb.201811109

Real-time imaging technology detecting single-molecule mRNAs enables detection of molecular dynamics of mRNA regulations including transcription, translation and mRNA localization.
Real-time imaging of mRNAs encoded ER-targeting protein labeled with fluorescence protein.
Translating mRNA is attached to the endoplasmic reticulum (ER) due to co-translational translocation. Untranslating mRNA is freely diffusing away into unfocused area.
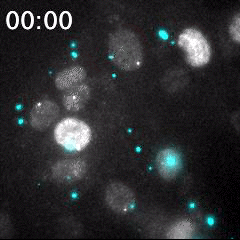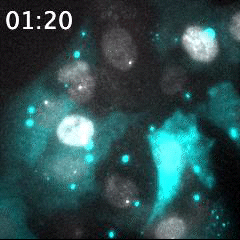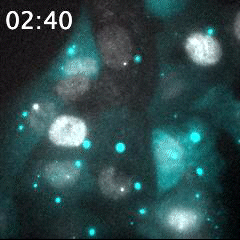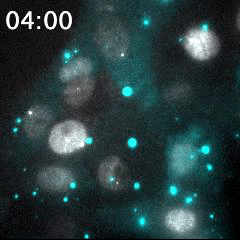
Real time imaging of transcribing mRNAs (Bright gray spots) in the nucleus (Gray) with Fluorescence labeled siRNA (Cyan) delivering using lipid nanoparticle (LNP). LNP before being delivered into the cells was identified as Bright cyan particles. Cyan fluorescence is spread in the entire cell area by fluorescence-labeled siRNA delivery into the cells. This imaging approach is useful for an evaluation of oligonucleotide-based drug delivery.
Further Information
Asst. Prof. Hanae Sato’s episode on the NanoLSI Podcast.




