Dynamic interplay between lateral diffusion and conformational states in a secondary transporter revealed by high-speed AFM
Researchers from the Nano Life Science Institute (WPI-NanoLSI) at Kanazawa University in collaboration with researchers from the University of Barcelona (Catalonia, Spain) published a high-speed atomic force microscopy study in Colloids and Surfaces B: Biointerfaces to directly visualize the dynamic behavior of lactose permease (LacY), a secondary active transporter in E. coli, within proteolipid sheets. The researchers revealed that LacY exhibits rapid lateral diffusion and undergoes substrate-induced conformational changes closely related to the membrane’s mechanical state. By combining real-time imaging of the protein and nanomechanical analysis of the lipid bilayers, the work demonstrates that the transporter’s function depends not only on static molecular structure but also on the interplay between protein mobility, the lipid environment, and electrochemical gradients.
Context
Secondary transporters, such as LacY, utilize ion gradients to drive substrate uptake; however, their dynamics in fluid membranes are still not well understood. While the structure and mechanism of LacY are well studied, its behavior in a native-like membrane environment is less known. HS-AFM provides the necessary temporal and spatial resolution to directly observe membrane protein mobility and conformational changes. Understanding these dynamic processes is crucial not only for membrane biophysics, but also for the engineering of biomimetic nanosystems and functional membrane-based devices.
Key Findings
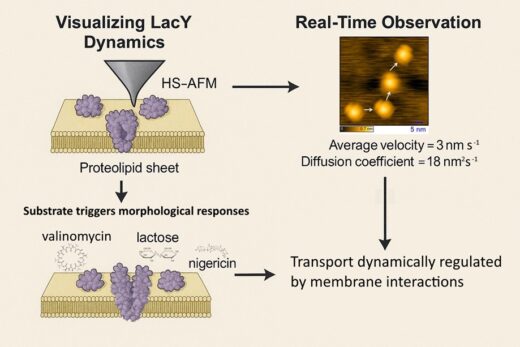
Lateral Diffusion: LacY appears embedded in E. coli lipid membranes mainly as dimers and diffuses at ~3 nm/s (D ~ 18 nm² s-1), enabling significant repositioning along the lipid bilayer during a transport cycle.
Conformational Changes: Lactose (natural substrate of the protein) binding slightly reduces LacY’s protrusion (height and volume), consistent with substrate-induced structural rearrangements.
Influence of Ion Gradients: Valinomycin enhances protrusion and suggests activation of lactose transport. Nigericin traps LacY in intermediate states by collapsing ion gradients.
Membrane Mechanics: The addition of lactose stiffens the lipid bilayer and increases adhesion forces, indicating that substrate and ions alter membrane nanomechanics.
Perspectives
The findings highlight that the function of LacY is complex and involves the dynamic interaction between protein mobility, conformational changes, and membrane mechanics. This challenges static perspectives on transport mechanisms and supports models where the biophysical properties of the surrounding lipid environment influences transport efficiency. The work also provides guidance for the design of biomimetic membranes or nanosystems where protein mobility is crucial for their function.
Article
- Title
- Dynamic interplay between lateral diffusion and conformational states in a secondary transporter revealed by high-speed AFM
- Author
- Oscar Domènech*, Xuan Kien Ngo, Adrià Botet-Carreras, Jordi H. Borrell
- Journal
- Colloids and Surfaces B: Biointerfaces
- Publication date
- Nov 21, 2025
- DOI
- 10.1016/j.colsurfb.2025.115282
- URL
- https://www.sciencedirect.com/science/article/abs/pii/S0927776525007891

