An Artificial Hepatocyte Growth Factor Mimetic Ameliorates Non-Alcoholic Steatohepatitis in Mouse Model
A research group led by Associate Professor Katsuya Sakai, Researcher Nichole Marcela Rojas-Chaverra, and Professor Kunio Matsumoto of the Cancer Research Institute and Nano-Life Science (WPI-NanoLSI) at Kanazawa University has developed a long-acting, subcutaneously injectable artificial hepatocyte growth factor (HGF) mimetic molecule using a fusion technology of cyclic peptides and protein engineering. They demonstrated in a mouse model that it improves liver fibrosis, lipid accumulation, and inflammation caused by non-alcoholic steatohepatitis (NASH). This research result provides an option for the development of NASH therapeutics as well as a technology for creating growth factor and cytokine mimetic molecules with improved pharmacokinetics.
NASH is a liver disease in which fat accumulates in the liver, leading to inflammation and fibrosis. It can lead to serious complications such as cirrhosis and liver cancer. In recent years, the number of NASH patients is estimated to be 25-30% worldwide due to the increase in obesity and diabetes, and the development of new therapies is urgently needed.
Hepatocyte growth factor (HGF)*1 is known to play an important role in hepatocytes regeneration and protection by binding to the c-Met receptor.*1 However, it has the challenges of a short half-life in blood and limited administration methods, despite its potential as a therapeutic agent for NASH.
In this study, the research group developed an HGF mimetic molecule by newly identified cyclic peptides that bind to the c-Met receptor using in vitro mRNA display method*2 and grafting it into the crystallizable region (Fc) of an immunoglobulin. This HGF mimetic molecule overcomes the pharmacokinetic drawbacks of HGF by having the properties of Fc, which prolongs its half-life in blood circulation and allows for subcutaneous administration. When the HGF mimetic molecule was subcutaneously administered once a week to mice with NASH induced by a choline-deficient high-fat diet, liver fibrosis, inflammation, and fat accumulation were significantly improved compared to the control group.
This research result provides a new option for NASH therapeutics and provides a technical basis for creating growth factor and cytokine mimetic molecules that expand the range of therapeutic applications by improving pharmacokinetics.
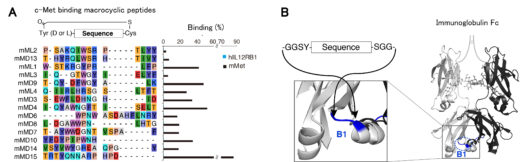
Figure 1: HGF mimetic molecule was developed by grafting the newly identified c-Met receptor-binding cyclic peptides (A) into the loop structures of immunoglobulin Fc (B).
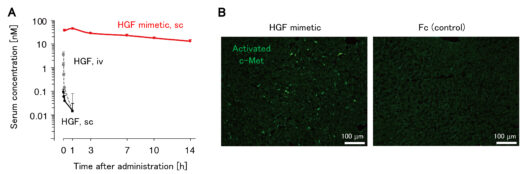
Figure 2: HGF mimetic molecule showed a significantly longer blood circulation time than HGF and allowed subcutaneous administration (A) and activated the c-Met receptor in hepatocytes in the liver (B).
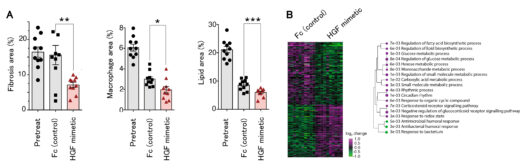
Figure 3: In a mouse NASH model, subcutaneous administration of the HGF mimetic molecule significantly improved liver fibrosis, inflammation, and fat accumulation compared to the control group (A), and recovery of liver function was observed, including an increase in the expression of metabolism-related gene groups (B).
Glossary
Article
- Title
- A cyclic peptide-grafted Fc with hepatocyte growth factor functionality ameliorates hepatic fibrosis in a non-alcoholic steatohepatitis mouse model
- Author
- Nichole Marcela Rojas-Chaverra, Ryu Imamura, Hiroki Sato, Toby Passioura, Emiko Mihara, Tatsunori Nishimura, Junichi Takagi, Hiroaki Suga, Kunio Matsumoto, and Katsuya Sakai* *Corresponding author
- Journal
- iScience
- Publication date
- Jul 2, 2024
- DOI
- 10.1016/j.isci.2024.110426
- URL
- https://www.cell.com/iscience/fulltext/S2589-0042(24)01651-1

