Mechanism of scorpion toxin inhibition of K⁺ channel elucidated using high-speed AFM
Abstract
Agitoxin-2 (AgTx2) from scorpion venom is a potent blocker of K+ channels. Here, we observed the binding dynamics of AgTx2 to the KcsA channel using high-speed atomic force microscopy. From the images obtained, single-molecule kinetic analyses revealed that the affinity of the channel for AgTx2 increased during persistent binding and decreased during persistent dissociation. We propose a four-state model with relevant rate constants. An induced-fit pathway was dominant and accelerated binding by 400 times.
Background
Cell membranes contain ion channels that regulate the permeation of various ions between the inside and outside of the cell. Ion channels are proteins, and in response to diverse stimuli, they allow specific ions to permeate. In this way the cell regulates the electric signals that form the basis of the function of muscles and the nervous system. Since a malfunction of ion channels causes a number of disorders such as myocardial infarction and epilepsy, it is important to understand the mechanism of action of molecules that hamper ion channel functions.
Scorpion venom has been found to contain more than 200 compounds that bind to K+ channels*1). Among them, AgTx2 is a peptide*2) consisting of 38 amino acid residues. Previous studies showed that by binding to the K+ channel from outside the cell membrane, AgTx2 blocks the K+ channel pore and inhibits K+ permeation. However, the detailed molecular mechanism remained unknown, e.g. whether the binding dynamics could be explained by a two-state model of association and dissociation.
Results
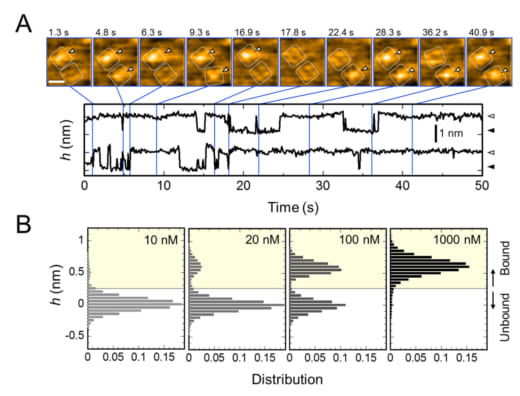
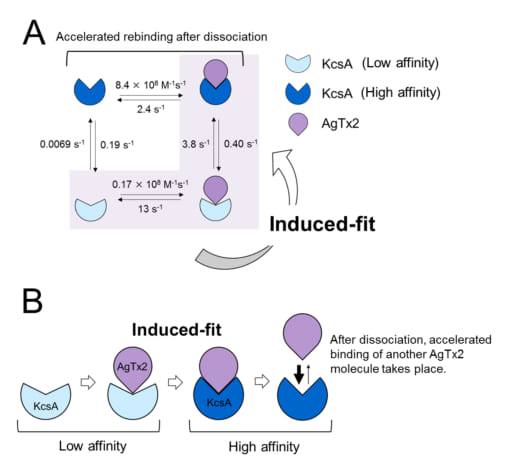
The present research team, including scientists from Kanazawa University, visualized the association and dissociation of AgTx2 with the K+ channel KcsA*3) using high-speed atomic force microscopy (HS-AFM)*4) and performed a detailed analysis of the dynamics (Figure 1). KcsA is a tetramer, its subunits forming a ring-like structure. Upon AgTx2 binding to the tetramer surface, the central part of the channel, i.e. the passage for K+, appeared swollen due to AgTx2 binding. The binding and dissociation of AgTx2 to the K+ channel could take place repeatedly and the height around the channel center changed accordingly (Figure 2). When the AgTx2 concentration in the solution was high, the binding probability was also high. Time courses of the binding and dissociation revealed that upon binding of AgTx2 to KcsA, the conformation of KcsA changed, which allowed AgTx2 binding more easily, and that even upon dissociation of AgTx2, another AgTx2 molecule could bind to KcsA very quickly; this is referred to as an induced-fit*5) mechanism. On the other hand, it was observed that, when sufficient time elapsed after AgTx2 dissociation, the conformation of KcsA changed back to the original, to which AgTx2 bound less readily. These results indicate that the binding dynamics cannot be explained with a simple two state model, i.e. binding and dissociation. It seemed that both the association and dissociation states of KcsA could be distinguished as at least two sub-states; a four state association model was therefore considered (Figure 3). When the reaction rates were calculated for a four-state model the rates obtained reflected those obtained experimentally. A simulation of the binding dynamics with this four-state model and the associated reaction rates, showed AgTx2 binding to be primarily via the induced-fit pathway. Further, it was found that the induced-fit accelerated AgTx2 binding by 400-fold. Thus, the mechanism of AgTx2 to efficiently inhibit the function of KcsA has been elucidated (Figure 3).
Future prospects
The HS-AFM observation technique and method used for the analysis of binding of the K+ channel and the peptide inhibitor can be applied to a wide variety of biological molecules. They can be applied to analyses of a ligand binding to its receptor as well as association of DNA and a DNA-binding protein. This study is expected to accelerate the elucidation of binding dynamics of various biological molecules.
Figure 1. Scheme of association and dissociation of potassium channel KcsA (Q58A/T61S/R64D) and AgTx2 and representative AFM images.
A. Association (binding) of KcsA and AgTx2.
B. Scheme showing a specimen for AFM observation.
C. Representative AFM images and with dimensions shown as white dotted lines. Bar, 2 nm.
Figure 2. Repetitive binding of AgTx2 to potassium channel KcsA and AgTx2 – concentration dependence of binding probability.
A. Time-lapse images of AgTx2 binding to and dissociation from the KcsA channels and time courses of the average height (h) (nm) around the center of the extracellular surface. Scale bar in the HS-AFM image, 5 nm.
B. Height histograms of h depending on AgTx2 concentration in the solution.
Figure 3. Binding dynamics model of AgTx2 and potassium channel KcsA.
A. Rate constants of reaction steps.
B. Accelerated binding of AgTx2 to potassium channel KcsA through induced-fit mechanism.
Glossary
Article
- Title
- High-speed AFM reveals accelerated binding of Agitoxin-2 to K+ channel by Induced-fit
- Author
- Ayumi SUMINO, Takashi SUMIKAMA, Takayuki UCHIHASHI, Shigetoshi OIKI
- Journal
- Science Advances
- Publication date
- Jul 3, 2019
- DOI
- 10.1126/sciadv.aax0495
- URL
- https://advances.sciencemag.org/content/5/7/eaax0495