Gaining insight into the molecular mechanisms behind squamous cell cancer
Abstract:
Researchers at Kanazawa University report in EMBO Reports a new molecular mechanisms regulating cellular fate of squamous cell carcinomas
Background
Squamous cell carcinoma (SCC) *1 is a lethal cancers arising from the stratified epithelia of skin, esophagus, cervix, as well as the head and neck tissues. Genomic analysis of SCCs identified genomic amplification of TP63 in up to 30% of tumors, with overexpression of its mRNA in the majority of SCCs.
ΔNp63α, one of the proteins encoded by TP63, has an important role in the epithelial development and maintenance. In SCCs, DNp63a functions as a key transcriptional regulator of different gene subsets in order to maintain or enhance malignant phenotypes. However, the mechanism controlling the nuclear transport of this protein, were, up to now, unclear.
Nucleoporins (NUPs) are a family of proteins building nuclear pore complexes (NPC) *2 and mediating nuclear transport across the nuclear envelope. Recent evidence suggests a cell-type-specific function for certain NUPs; however, the significance of NUPs in SCC biology remains unknown.
Results
In the present study, Hazawa et al. show that one particular nucleoporin, nucleoporin 62 (NUP62), is highly expressed in stratified squamous epithelia, and is further elevated in SCCs. They further demonstrate that depletion of NUP62 inhibits proliferation and augments differentiation of SCC cells, suggesting NUP62 is required for preventing epidermal differentiation of SCCs. The impaired ability to maintain the undifferentiated status is associated with defects in ΔNp63α nuclear transport. Finally, they unmasked the detailed traffic machinery where the pro-differentiation Rho kinase (an enzyme that catalyzes the transfer of phosphate groups) inhibits the nuclear transport of ΔNp63α by reducing the interaction between NUP62 and ΔNp63α.
Future prospects
This study demonstrates the role of NUP62 regulating cellular fate of SCCs through ΔNp63α nuclear transport. However, whether these NUPs regulates cell identity in different tissues (or in other types of cancer cells) is still an open question. As the authors comment in the paper: “Our finding of convertible trafficking activity of NUP62 highlights the potential for therapeutic targeting of nuclear transport of this oncogene.”
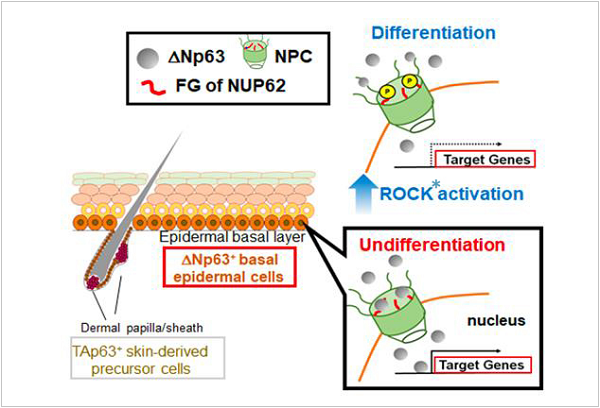
Figure: Hypothetical model of NUP62 action in regulating cell fate.
* Rho-associated protein kinase (ROCK)
A kinase (that is, an enzyme that catalyzes the transfer of phosphate groups) involved in regulating the shape and movement of cells by acting on the cytoskeleton.
Glossary
Article
- Title
- ROCK‐dependent phosphorylation of NUP62 regulates p63 nuclear transport and squamous cell carcinoma proliferation
- Author
- Masaharu HAZAWA, De-Chen LIN, Akiko KOBAYASHI, Yan-Yi JIANG, Liang XU, Firli Rahmah Primula DEWI, Mahmoud Shaaban MOHAMED, Hartono, Mitsutoshi NAKADA, Makiko MEGURO-HORIKE, Shin-ichi HORIKE, H. Phillip KOEFFLER, Richard W. WONG
- Journal
- EMBO Reports
- Publication date
- Dec 7, 2017
- DOI
- 10.15252/embr.201744523
- URL
- http://doi.org/10.15252/embr.201744523

