High speed in-liquid atomic force microscopy (HS-AFM): Direct visualization of proteins in action
Unique HS-AFM instrumentation enables direct visualization of the structure and dynamics of protein molecules in liquids

Toshio Ando, Professor, Nano Life Science Institute (WPI-NanoLSI), Kanazawa University
Toshio Ando is a biophysicist whose curiosity about how proteins function led him to develop high speed atomic force microscopy (HS-AFM). “Ever since my undergraduate days I have been intrigued by proteins because nearly all biological phenomena depend on them,” says Ando. “Muscle contraction, cell division, and so on, are biological processes that depend on proteins. So, I wanted to find a way of directly observing the dynamics of proteins. This curiosity led to the invention of the in-liquid HS-AFM. It is now a de-facto technology in labs all over the world.”
The major conventional methods for studying proteins are structural analysis methods, such as X-ray crystallography, electron microscopy, and NMR. Furthermore, bimolecular dynamics are typically studied with fluorescent microscopy by tagging target molecules with fluorescent probes. The former structural methods only yield ‘snapshots’ and in fluorescence microscopy, the actual target molecules are not visible–so it is not a direct method for observing the dynamics of biomolecules.
To overcome the limitations of conventional technology, Ando started his research on the instrumentation of HS-AFM in 1993 and eventually reported on the establishment of HS-AFM in 2008 [1]. Underscoring the importance of this invention, this system has been used to visualize a wide range of biological processes including the spectacular dynamics of myosin V walking along actin filaments [2] (Fig.1).
Although HS-AFM has proven an excellent technology for visualizing the dynamics of purified protein molecules in liquid environments, this method can lead to deformation of eukaryotic membranes due to contact between the AFM tip and sample, which leads to blurred and low-resolution images. To resolve this problem, Ando and colleagues are developing non-invasive high-speed scanning ion-conductance microscopy (HS-SICM) for high-speed and high-resolution imaging of biomolecules in liquids.
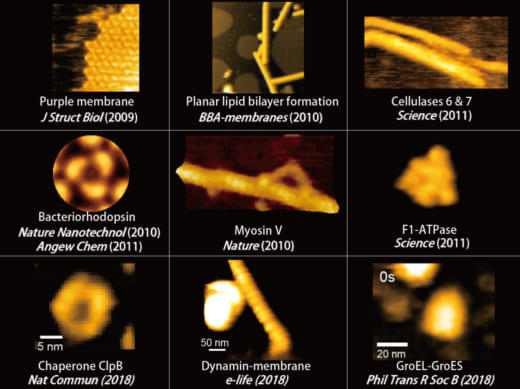
Fig. 1: Protein molecules filmed with HS-AFM during their functional activity.

Bacteriorhodopsin
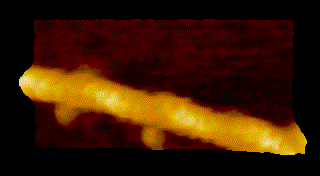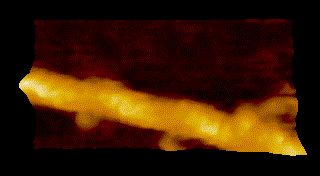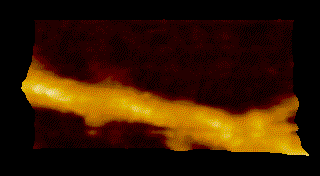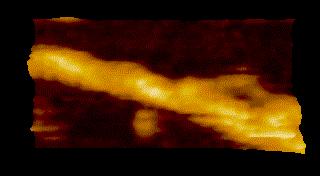
MyosinV
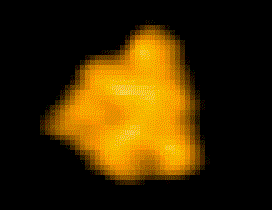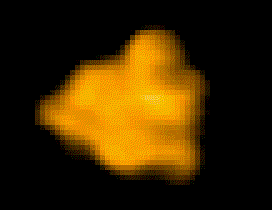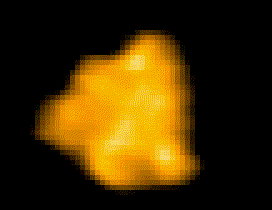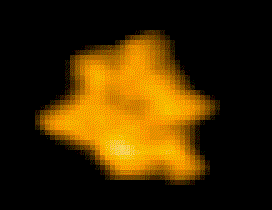
F1-ATPase

ClpB
Realization of a functional HS-SICM necessitates the development of SICM probes with tip pore diameters of 1-2 nm and wall thickness less than 1 nm. Fig 2 is an illustration of a CNT pipette consisting of a lipid bilayer integrated with the end of a nano-pipette. “We have successfully inserted carbon nanotubes into lipid bilayers in nano-pipettes and observed an ion current through the CNT,” says Ando. “This is promising for our long term goals. We are also developing a HS-SICM system using the CNT nano-pipette probes.”
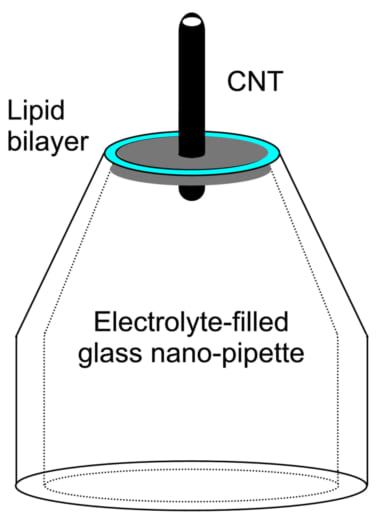
Fig. 2: Carbon nanotube (CNT) pipette.
Ando reported the development of the world’s fastest HS-AFM for imaging biological samples in liquids in 2008. His attention is now focused on high resolution SICM with the potential of opening new areas of research in the life sciences.
Research highlights
Toshio Ando has published many internationally acclaimed, high impact papers during his illustrious career. The first report on the HS-AFM in 2001 [3], which has so far been cited more than 600 times, is a highly instructive description of the limits of conventional AFM systems and how Ando designed and built the HS-AFM for liquid environments.
Seminal publications on the applications of HS-AFM include Nature 2010, dynamics of myosin V [2]; behavior of photo-activated bacteriorhodopsin [4]; hydrolytic efficiency and translational motion of cellulases on cellulose surface [5]; rotational propagation of chemical and structural states in rotor-less F1-ATPase [6]; dynamic structural changes of molecular chaperone ClpB during its ATPase reaction [7]; and others (see Fig.1).
References
- Ando et al., “High-speed atomic force microscopy for nano-visualization of dynamic biomolecular processes,” Prog. Surf. Sci. 83, 337–437, (2008).
DOI: 10.1016/j.progsurf.2008.09.001 - Kodera, D. Yamamoto, R. Ishikawa, and T. Ando, “Video imaging of walking myosin V by high-speed atomic force microscopy”, Nature 468, 72–76 (2010).
DOI: 10.1038/nature09450 - Ando, N. Kodera, D. Maruyama, E. Takai, K. Saito, and A. Toda, “A High-speed atomic force microscope for studying biological macromolecules, Proc. Natl. Acad. Sci. USA98, 12468–12472 (2001).
DOI: 10.1073/pnas.211400898 - Shibata, H. Yamashita, T. Uchihashi, H. Kandori, and T. Ando, “High-speed atomic force microscopy shows dynamic molecular processes in photo-activated bacteriorhodopsin”, Nat. Nanotechnol. 5, 208–212 (2010).
DOI: 10.1038/nnano.2010.7 - Igarashi, T. Uchihashi, A. Koivula, M. Wada, S. Kimura, T. Okamoto, M. Penttilä, T. Ando, and M. Samejima, “Traffic jams reduce hydrolytic efficiency of cellulase on cellulose surface”, Science 333, 1279–1282 (2011).
DOI 10.1126/science.1208386 - Uchihashi, R. Iino, T. Ando, and H. Noji, “High-speed atomic force microscopy reveals rotary catalysis of rotorless F1-ATPase, Science 333, 755–758 (2011).
DOI: 10.1126/science.1205510 - Uchihashi, Y. Watanabe, T. Yamasaki, H. Watanabe, T. Maruno, K. Ishii, S. Uchiyama, C. Song, K. Murata, R. Iino, and T. Ando, “Dynamic structural states of ClpB involved in its disaggregation function”, Nat. Commun. 9, 2147 (12 pp) (2018).
DOI: 10.1038/s41467-018-04587-w

