Frontiers of supramolecular chemistry: Advances in macrocyclic materials for life sciences
Serendipitous discovery of pillar[n]arenes by Tomoki Ogoshi in 2008 has led to unprecedented advances in nanoscale molecular recognition and guest-host chemistry

Tomoki Ogoshi, Professor, Nano Life Sciences Institute (WPI-NanoLSI), Kanazawa University
Macrocyclic compounds are cyclic chemical structures such as ‘crown ethers’ that contain large rings with potential applications that include molecular recognition. “My interest in macrocyclic materials was triggered by a case of serendipity,” says Tomoki Ogoshi, principle investigator (PI) in the supramolecular chemistry group of the Kanazawa University WPI- NanoLSI project. “We were preparing phenol-paraformaldehyde resins when we discovered the formation instead of unique, pillar-like structures that we later named pillar[n]arenes [1]. I am working with colleagues at the NanoLSI to use them as high-resolution nano-probes to detect cancer cells.”
The pillarenes consist of benzene units connected by methylene linkage at para-positions, which is important because the existence of para-bridge linkages leads to structures with very high symmetrical pillar-shapes, which is different from typical cyclic compounds (Fig.1).
The report of the discovery of pillar[n]arenes in 2008 has led to a tremendous increase in publications based on these structures. “I have produced a ‘pillararene chemists world map’ to keep track of the tremendous proliferation of research using this materials,” says Ogoshi. “In China there are over 30 individual groups; Europe over 10 groups; other parts of Asia in including me, about 5 groups; and about five groups in the USA. Nobel Laurette Prof. Stoddart also uses pillararenes. So, this field is very hot and exciting. The pillararene community is huge. There have been more than 500 peer reviewed papers published since 2008 [2].”
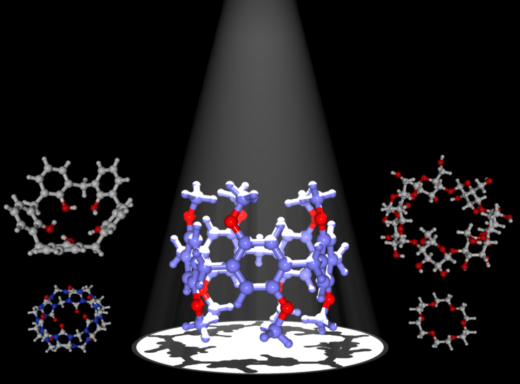
Fig.1: Pillararenes synthesized by Tomoki Ogoshi in 2008 [1].
Areas of research include nanoscopy for looking at intracellular dynamics; nanomapping and capturing specific molecules based on intermolecular interactions; molecular design with guest-host arrays; distribution of oncometabolite in cells; and detection of nicotinamide biomarkers for cancer cells.
Research highlights
Ogoshi and his colleagues reported the first synthesis of para-bridged pillar-shaped pillar[5]arenes in 2008 [Ref 1 and Fig. 2]. The procedure consists of condensation of 1,4-dimethoxybenzene(DMB) with Lewis acids. The existence of reactive hydroxyl groups at both ends enables the structures to be easily functionalized opening up the possibilities of wide ranging applications based on their use as hosts.
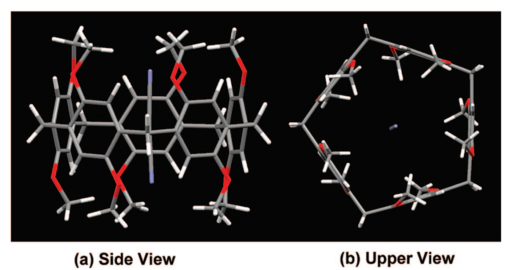
Fig.2: Upper and side views of the structure of pillar[5]arene.
Review of the basic properties of potential pillar-shaped macrocyclic hosts pillar[n]arenes and potential applications [2]. Here, Ogoshi and his co-authors provided detailed insights into the synthesis of pillar[n]arenes for newcomers to this field and well as forecasting potential applications for host-guest chemistry in supramolecular polymers and electronic materials.
Fig 3: Review on the properties and applications of pillar[n]arenes [2]
References
- Tomoki Ogoshi, Suguru Kanai, Shuhei Fujinami, Tadaaki Yamagishi, and Yoshiaki Nakamoto, “para-Bridged Symmetrical Pillar[5]arenes: Their Lewis Acid Catalyzed Synthesis and Host–Guest Property”, J. Am. Chem. Soc. 130, 5022–5023, (2008).
DOI: 10.1021/ja711260m - Tomoki Ogoshi, Tadaaki Yamagishi, and Yoshiaki Nakamoto, “Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry”, Rev. 116, 7937−8002 , (2016).
DOI: 10.1021/acs.chemrev.5b00765
Further information
* Dimethoxypillar[5]arene is commercially available from Tokyo Chemical Industry CO., LTD.
Tokyo Chemical Industry CO., LTD
http://www.tcichemicals.com/en/jp/index.html
Dimethoxypillar[5]arene
http://www.tcichemicals.com/eshop/en/jp/commodity/D4471/

