Frontiers of supramolecular chemistry: Strategies for on-demand guest exchange
Innovative approaches to trap and release guest species from nanomolecular cages for biomolecular sensing and nanoendoscopy

Shigehisa Akine, Professor, Nano Life Sciences Institute (WPI-NanoLSI), Kanazawa University
Shigehisa Akine is a principle investigator (PI) in the supramolecular chemistry group of the Kanazawa University WPI-NanoLSI project. “Supramolecular chemistry is the study of mechanisms governing interactions between molecules,” says Akine. “Scientifically, these are known as noncovalent interactions, which are contrast to covalent bonding that is responsible for holding individual atoms together in a molecule.”
One of the main goals of his research is to chemically synthesize ‘molecular cages’, e.g., host molecules with a three-dimensional cavity, in order to capture specific target ‘guest molecules’. “We are designing host molecules using oligo(salen)–metal structures,” explains Akine. “We chemically design these host compounds to have cap functions enabling the control of guest targets to be either confined inside the host or be released. This ability to switch the cap on or off is important for many applications in the WPI-NanoLSI project including the nanoendoscopy.”
Molecular hosts with switchable cap functions can be envisioned as being like the caps of containers and plastic bottles used in everyday life: open the cap to remove the contents and close it to keep the contents inside the container (Fig.1). On the nanometer scale the molecular hosts can be used to store, transport, and release guest molecules for wide ranging applications related to molecular recognition and nano-molecular machines.
Akine and his colleagues have designed and demonstrated the potential of innovative molecular cages based on oligo(salen)–metal structures that enable open/close functions to admit and release guest species. In their approach they introduce or remove bridging ligands into the metal–organic cage structures to control the uptake and release of guests. This is one of several strategies for on-demand guest exchange.
Specific examples of their approach include a tricobalt (III) metallocryptand structure with diamine ligands at the aperture of the molecular cage that selectively recognizes a cesium ion, but importantly, the uptake rate is 2000 times slower than for the corresponding ‘open’ cage [1]. This closed cage structure demonstrates the effectiveness of this approach to ‘close’ the host and significantly reduce the rate of guest uptake.
In another procedure, anion-capping of a macrocyclic metallohost enables control of guest entering and exiting of the oligo(salen)–metal structures [2]. The unique feature here is that triggering guest exchange depends on the choice of anion caps from a very stable kinetically trapped state. “We successfully controlled complicated processes for ion uptake and release by using only cobalt metallohost,” says Akine. “This simple but effective approach will be important to fabricating nanocages for sensing and other applications.”
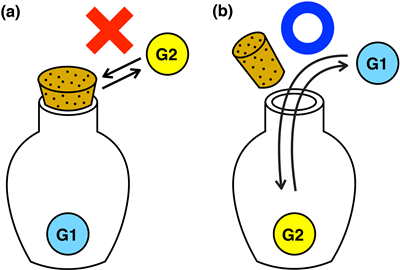
Fig.1: Macroscopic equivalent of nanometer sized ‘host’ molecular cages and their ‘guests’. (a) The object is trapped inside the bottle and cannot be removed with a tight stopper. (b) A looser stopper enables the object to be removed.
Research highlights
Recent research papers include a topics on “regulatory enzyme”-like strategy of helicity inversion published in Scientific Reports [3] and the interaction of polycyclic aromatic hydrocarbons that can be used for recognition of planar aromatic guests, published in New Journal of Chemistry [4].
References
- S. Akine etal. “Metallo-molecular Cage That Can Close the Apertures with Coordination Bonds”. J. Am. Chem. Soc. 139, 4631, (2017).
doi:10.1021/jacs.7b00840 - Y. Sakata, C. Murata, and S. Akine, “Anion-capped metallohost allows extremely slow guest uptake and on-demand acceleration of guest exchange”. Nat. Commun. 8, 16005, (2017).
doi:10.1038/ncomms16005 - S. Sairenji et al, “Response speed control of helicity inversion based on a “regulatory enzyme”-like strategy”, Sci. Rep. 8, 137, (2018).
doi:10.1038/s41598-017-16503-1 - S. Akine et al, “A novel graphite-like stacking structure in a discrete molecule and its molecular recognition behavior”, New J. Chem. 42, 9369, (2018).
doi:10.1039/C8NJ01315B

