Thermodynamic cell engineering to visualize and control cellular activities

Satoshi Arai, Associate Professor, Nano Life Science Institute (WPI-NanoLSI), Kanazawa University
Thermodynamic cell engineering to visualize and control cellular activities
Satoshi Arai postulates that heat may offer a universal means of manipulating cellular functions because biological processes such as chemical reactions, membrane fluidity, and structural dynamics of biomacromolecules are governed by temperature.
“Heat is critical for the existence of life on Earth,” says Arai. “As the philosopher Hippocrates said, ‘Give me the power to create heat, and I shall cure any diseases.’ In my research I am visualizing energy flow inside cells to control cell functions via thermodynamic engineering.”
Visualizing energy flow in living cells
The organic chemical adenosine triphosphate (ATP) is the source of energy that powers a wide range of processes in living cells. ATP is the ‘energy currency’ for storing and transferring energy within cells. While a cell drives cellular activities using the stored ATP, the part of energy is dissipated as heat, leading to the temperature rise inside a cell. For deeper understanding of the energy flow, it is necessary to capture the spatio-temporal dynamics of ATP and temperature at cellular level. “In our research, we visualize temperature change in cells using fluorescent thermometers based on temperature sensitive dyes,” explains Arai. “We have developed protocols to place the thermometer to specific targets in cells. The important point is that the dyes enable thermometry at ‘zero distance’ from the source of heat. So this method contributes to find out hot spots at high spatial resolution.”
Arai and his co-workers have reported on the visualization of heat production in a wide variety of cells using fluorescent thermometers [Fig. 1]. Examples include: endoplasmic reticulum, mitochondrion, and lipid bilayers [1,2]. “Our report on thermal imaging of thermogenesis in Brown Adipose tissues offers an excellent tool for screening nutrients and drugs to promote the generation of heat for melting fat” says Arai. “We can also produce RGB fluorescent ATP sensors and visualize the ATP dynamics as the other key element for the cellular energy flow [3].”
Thermal control of cellular functions using nanoheaters
Conventional heating of cells is based on direct irradiation of tissues with near-infrared (NIR) lasers with wavelengths at 980 nm to 1480 nm—the peaks for the absorption light by water. However, this approach is not suitable for high precision, local heating at the subcellular level. Recently, Arai and his colleagues developed an innovative approach for localized heating and temperature sensing of cells [Fig. 2]. “Our solution is to embed a photothermal dye that generates heat when irradiated with NIR lasers into a ~200 nm diameter nanoparticle made of organic polymer matrix,” says Arai. “This ‘nanoheater’ is also embed with a temperature sensing dye to monitor the heat generated by the nanoheater. This approach enables nanoscale heating and nanoscale thermal imaging of cells.”
Arai has studied the effect of nanoheaters on cell activity. The rapid cell death spread from the heat spot generated by a nanoheater and only started when the temperature was above a certain temperature, with no cell death below this temperature, and the mitochondria disintegrated into small pieces due to the heat shock.
Plans for the future
In the short term, Arai will focus on developing practical biodegradable nanoheaters with high biocompatibility. Mid term plans include realization of therapeutic devices for mild heat therapy for cancer, metabolic diseases, and muscle dysfunction; and the development of high precision standardized thermometers.
“There are many challenges ahead, both industrial and academic,” says Arai. “For an academic challenge will be to answer the question as to the meaning of a localized temperature rise in the context of biomolecules. Also, as part of the WPI-NanoLSI I would like to develop chemical tools to unveil the mechanism of biological phenomena at atomic scale through the collaboration studies with the Bio-AFM and supramolecular chemistry teams.”
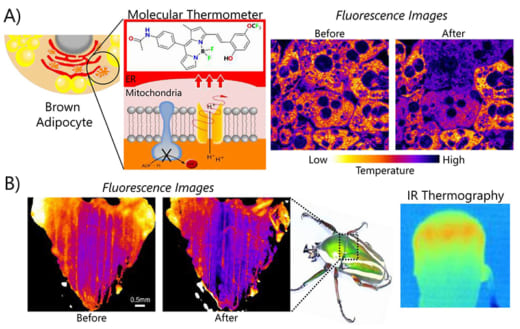
Fig. 1: Temperature mapping from organelles to animals. Visualization of thermogenesis in brown adipocytes (A) and the flight muscle of beetle (B). Fluorescence images indicate temperature mapping before and after heat production.
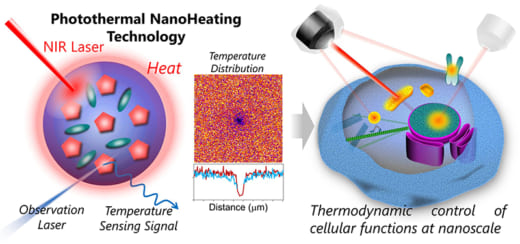
Fig. 2: Nanoheating technology using photothermal materials and fluorescent thermometers.
References
- A Molecular Fluorescent Probe for Targeted Visualization of Temperature at the Endoplasmic reticulum, *Satoshi Arai, Sung-Chan Lee, Duanting Zhai, Madoka Suzuki, Young Tae Chang, Scientific Reports. 4, 6701 (2014).
- The ABC Guide to Fluorescent Toolsets for the Development of Future Biomaterials, Ferdinandus, *Satoshi Arai, Front. Bioeng. Biotechnol. 7:5. (2019).
- RGB-Color Intensiometric Indicators to Visualize Spatiotemporal Dynamics of ATP in Single Cells, *Satoshi Arai, Rlkus Kriszt, Kazuki Harada, Liang-Sheng Looi, Shogo Matsuda, Devina Wongso, Satoshi Suo, Shoichi Ishiura, Yu-Hua Tseng, Michael Raghunath, Toshiro Ito, Takashi Tsuboi, and Tetsuya Kitaguchi, Angew. Chem. Int. Ed. 57, 10873-10878 (2018).

