Genetic mouse models: Knock-out innovations for cancer prevention and treatment
Nanoscale visualization of the true nature of cancerization and malignant progression mechanisms

Masanobu Oshima, Professor, Nano Life Sciences Institute (WPI-NanoLSI), Kanazawa University
Masanobu Oshima is internationally acknowledged for his contributions to research on cancer biology. “To-date I have worked with colleagues on developing genetic mouse models to study colorectal and gastric cancer,” says Oshima. “Our models have led to many breakthroughs.”
Examples of Oshima’s research include the development of a knock-out mouse model using mouse embryonic stem cells for intestinal tumors in 1995 [1] and the suppression of intestinal polyposis by COX-2 disruption that showed COX-2 to be a target for cancer prevention reported in Cell [2]. “Our report on new therapeutic compounds for colorectal polyposis and cancer in the 1996 paper in Cell has been cited more than 2000 times,” explains Oshima. “It opened up new roads for the treatment of cancer in the years that followed. Now, there are reports in the USA aspirin can prevent cancer. We have come a long way.”

Fig.1 Knock-out mice used in Ref.1
More recently, Oshima and his colleagues reported that gastric cancer is associated with inflammation, where they developed gastric cancer models by activation of oncogenic Wnt and inflammatory COX-2 [3]. This was the “first gastric cancer model that recapitulates human cancer,” says Oshima.
“In the NanoLSI project we will study the true nature of ‘cancerization’ and ‘malignant progression’ mechanisms at the nanometer level to develop new methods for diagnosing and treating cancer,” says Oshima. “We will use HS-AFM and SICM to visualize abnormalities in cell structures due to transformation and malignant progression of tumor cells and dynamic changes of pharmacokinetics and conduct metabolic measurements with new nanoprobes that are being developed by scientists at the NanoLSI.”
Specific plans include the examination of the structures of the surfaces of cells, cytoskeletons at the nanometer scale, to visualize how they are modified in malignant progression such as epithelial–mesenchymal transition (EMT). This will make significant contributions to understanding the progression mechanism of cancer cells.
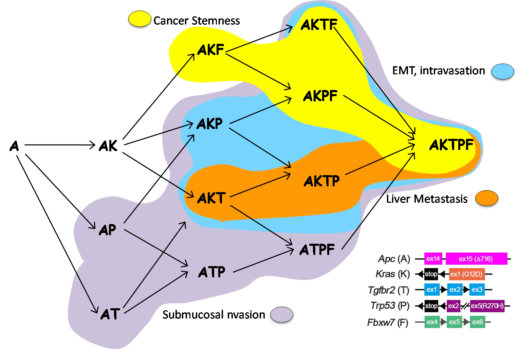
Early experiments are promising with Oshima and his group having determined genotype-phenotype relationship during colon cancer progression. “Our cell collection including A, AK, AKT, AKP, AKTP is unique and significantly different from commercially available cancer cells that contain large numbers of mutations,” emphasizes Oshima. “Currently, our understanding of EMT is based only on static imaging of frozen samples using scanning and transmission electron microscopy. We want to pioneer real time visualizing for research on cancer.” This research is being conducted by using ogoninal organoid cell lines at Kanazawa University (A/Ak/AKP/AKPT) ranging from benign to malignant.
Another topic of research is the examination of the tetramer complex formation of mutant p53. This is an important subject because We found Oshima and his team recently discovered that mutant p53 is nuclear accumulated in mutant homozygous but not in heterozygous cells [Nakayama, et al, Oncogene, 2018]. It is not clear how mutant p53 localization is regulated and whether the regulation is related to complex formation.
Research highlights
In 2018 Oshima and his group published their findings on the construction of multiple gene mutant mice (combinations of A, K, T, P, F), and linked genotype-phenotype relations. These mouse models and allografts of tumor-derived organoids showed the progression processes of colon cancer. These results are important for the prevention of colorectal cancer metastasis by targeting signaling pathways that control specific progression processes [Nakayama, et al, Oncogene, 2017; Sakai, et al, Cancer Res, 2018].
References
- Oshima et al. “Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene”, PNAS, 92, 4482-4486 (1995).
DOI: 10.1073/pnas.92.10.4482 - Oshima et al. “Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2)”, Cell, 87, 803-809, (1996).
- H Oshima et al. “Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway”, Gastroenterology, 131, 1086-1095, (2006).
- Sakai, et al. “Combined Mutation of Apc, Kras, and Tgfbr2 Effectively Drives Metastasis of Intestinal Cancer”, Cancer Res, March 2018.
DOI: 10.1158/0008-5472.CAN-17-3303

Reconstituted tumors using organoids.
Fig. 2: Evolution of CRC malignant progression [Ref.1] (left) and reconstituted heterogeneity of CRC by AKTP (green) and AP (red) tumor cells (right).

