Tissue regeneration and cancer progression: Hepatocyte growth factor and MET receptor for theranostics and drug discovery

Kunio Matsumoto, Professor, Nano Life Sciences Institute (WPI-NanoLSI), Kanazawa University
Kunio Matsumoto is a biochemist who has spent his career uncovering the mysteries of cancer progression, tissue regeneration, and drug discovery. “My education for my bachelor’s and master’s degree at Kanazawa University, and doctor’s degree at Osaka University was within a university laboratory with almost no exposure to clinical research in a medical environment,” says Matsumoto. “But I was employed by clinical department in Osaka University, though I was not medical doctor. At that time, I could not say I was lucky, but now I can say I was very lucky to have the opportunity of working at University Hospital and saw firsthand how doctors treat patients in the real-world of medicine. I remember doctors treating dermatological diseases and a four years old boy with a hole in his forehead. It is a quite unique that a basic researcher works in a clinical department. It was like an transdisciplinary experience at a young age. This was when I decided that I wanted to do medically related research to help people. These were the first steps along my research on growth factors, their receptors for regenerative medicine and drug discovery.”
Growth factors are proteins that pair up with receptor molecules to ‘signal’ cells to grow— a process referred to as cell division and proliferation. Matsumoto’s research is centered on studying the interaction between hepatocyte growth factor (HGF) and the MET receptor tyrosine kinase, that binds with HGF and transduces its signals across cell membranes (Fig. 1).
Experimental research by Matsumoto and colleagues has yielded new insights into the role of HGF in two-pronged approach toward tissue regeneration and cancer progression (metastasis and drug resistance) (Fig. 2). “In 1991 [1] we reported that HGF induced tubulogenesis—the formation of tubular tissues during organ formation—for kidney construction and reconstruction.” explains Matsumoto. “HGF was the only growth factor that induced tubulogenesis. Subsequently, we found that HGF promoted survival of neurons. HGF showed therapeutic actions in experimental disease models, including kidney disease, ALS, and spinal cord injury. We realized the tremendous potential of HGF for treating kidney disease, ALS, and spinal cord injuries.”
Based on these promising findings, Matsumoto and his colleagues launched Kringle Pharma, Inc. [2]. In 2019, phase-I/II clinical trial of recombinant HGF protein for treatment of patients with spinal cord injury was completed, which demonstrated its safety and efficacy. Besides, phase-II clinical trial of the one with ALS is ongoing. Matsumoto says, “I want to perform helpful work for patients, as a scientist working on basic research.” In the past few years, Matsumoto has extended his research through transdisciplinary collaborations: one is macrocyclic peptide technology and another is high-speed atomic force microscopy (HS-AFM) technology. In collaboration with chemical biotechnology, he succeeded in discovering artificial HGF, a synthetic macrocyclic peptide capable of activating MET in a very similar way to HGF (Fig. 1, right) [3]. Recently, his group identified HGF-inhibitory peptide-8 (HiP-8) [4]. HiP-8 is a macrocyclic peptide that binds to and inhibits HGF. HiP-8 seems to be applicable to cancer diagnosis and therapeutics.
The two main areas of research being undertaken at the NanoLSI are clarifying the dynamics and structure of biological receptor activation, and drug discovery by macrocyclic peptides for regenerative medicine and cancer diagnosis/therapeutics. Matsumoto has been collaborating with SPM specialists at the NanoLSI and obtained the first direct visual images of the structural dynamics of MET activation.
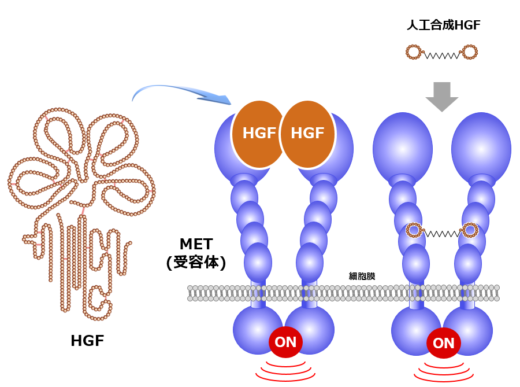
Fig.1: Outline structures of HGF, MET receptor and artificial HGF.
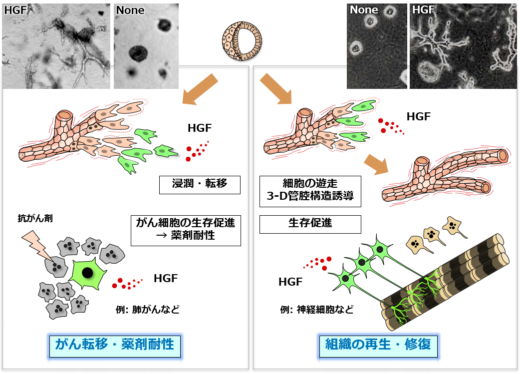
Fig.2: Two-pronged roles of HGF in tissue regeneration and cancer progression.
研究ハイライト
Important findings for targeting and inhibiting HGF include the discovery of HGF-inhibitory Peptide-8 (HiP-8) that binds to HGF and inhibits it. In collaboration with Dr. Shibata (NanoLSI), high-speed AFM visualization of the interaction between HiP-8 and HGF showed inhibition of dynamic domain movement in HGF by HiP-8 [4]. Likewise, high-speed AFM visualization of HGF-MET interactions revealed a new mechanism for MET receptor activation [unpublished].
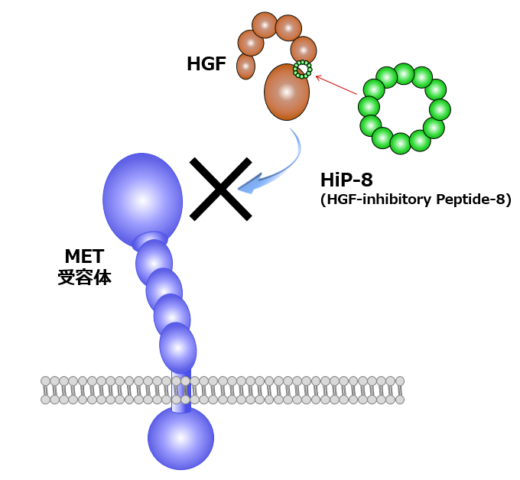
Fig. 3: Outline of HiP-8 action.
参考文献
- R. Montesano, K. Matsumoto, T, Nakamura, L. Orci, “Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor”, Cell 67, 901-8, (1991). doi.org/10.1016/0092-8674(91)90363-4
- Kringle Pharm, Inc. http://www.kringle-pharma.com/
- K. Ito et al, “Artificial human Met agonists based on macrocycle scaffolds”, Nature Commun, 6 6373, (2015).
- K. Sakai, T. Passioura, H. Sato, K. Ito, H. Furuhashi, M. Umitsu, J. Takagi, Y. Kato, H. Mukai, S. Warashina, M. Zouda, Y. Watanabe, S. Yano, M. Shibata, H. Suga & K. Matsumoto, “Macrocyclic peptide-based inhibition and imaging of hepatocyte growth factor”, Nature Chemical Biology, 15, 598–606 (2019)

