Scanning electrochemical microscopy (SECM): time rapse chemical mapping and identification of living cells
SECM enables quantification of molecules that play important roles in biological processes

Yasufumi Takahashi, Professor, Nano Life Sciences Institute (WPI-NanoLSI), Kanazawa University
“I am developing scanning ion conductance and electrochemical microscopy for chemical mapping,” says Yasufumi Takahashi. “Electron microscopes only enable imaging of dry samples. We want to image the dynamic properties of cells. So we have developed nanoelectrodes embedded in glass and carbon that are scanned over living cells in liquid environments to map their chemical distributions. This technology gives topographic and chemical information that is important to identify molecules.” Takahashi is collaborating with researchers at Imperial College, London on this research.
Time lapse Scanning Ion Conductance Microscope (SICM)
Scanning ion conductance microscopy (SICM) involves scanning a glass pipette with a nanometer diameter (typically 50 to 100 nm) orifice over biological samples in liquids. Information about the topography and charge distribution of surfaces and interfaces is obtained by analyzing fluctuations in the current flowing through a circuit that includes the gap between the orifice and sample. “The resolution of SICM is approximately 20 nm,” says Takahashi. “The major breakthrough has been to reduce the time to obtain one frame from 30 minutes to between 18 s. This is more than 100 times faster than conventional SICM systems.”
SICM combined with Fluorescence Resonance Energy Transfer (FRET) yields exceptional images of receptors on the surface of heart muscles and in conjunction with localized patch-clamp for recording “presynaptic ion channels”.
Scanning Electrochemical Microscopy (SECM)
This is another variation of scanning probe microscopy where a microelectrode consisting of a metallic wire encapsulated in glass cladding is scanned of samples yielding electrochemical information, such as oxygen concentration fluctuations, of the surfaces. Notably, SECM measurements monitor variations in current at the electrodes and the resulting images of living cells are much clearer than those obtained with optical microscopes.
“The SECM offers wide temporal resolution,” says Takahashi. “We have made milli-second measurements of neurotransmitters; taken images over tens of seconds of respiration activities of cells during drug sensitivity testing; and visualized protein (enzyme) expression process that take days.” Furthermore, the SECM not only allows imaging of biological processes, but it is also possible to trigger reactions by “delivering” chemical to cells and biomolecules.
Fabrication of nanoelectrodes for SECM
Miniaturization of the electrodes is one of the major issues for Takahashi and his colleagues [1-3]. “Currently, microelectrodes are made by fusing platinum wires into glass with the resulting probes having diameters of approximately 20 microns. In our approach, we used butane gas nanotubes to fabricate SECM electrodes. We have achieved nanoelectrodes with diameters of 13 nm. It’s a major breakthrough.”
SECM technology is expected to find applications in monitoring biological mechanisms using un-labeled molecules; determining the identity of molecules being imaged; and imaging synapses in neurological processes.
Research highlights
A selection of papers includes, “multifunctional nanoprobes for nanoscale chemical imaging and localized chemical delivery at surfaces and interfaces”, Angew. Chem. Int. [1], and “Topographical and electrochemical nanoscale imaging of living cells using voltage-switching mode scanning electrochemical microscopy”, PNAS [2].
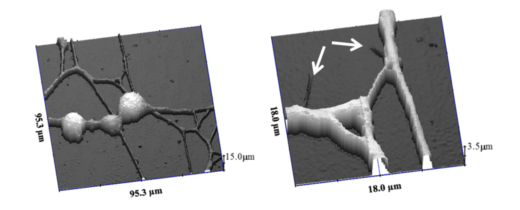
SECM images of neurons [1]
References
- Takahashi. et al. “Multifunctional Nanoprobes for Nanoscale Chemical Imaging and Localized Chemical Delivery at Surfaces and Interfaces”, Angew. Chem. Int. Ed. 50, 9638-9642 (2011).
- Takahashi, et. al., “Topographical and electrochemical nanoscale imaging of living cells using voltage-switching mode scanning electrochemical microscopy”, Proc. Natl. Acad. Sci. U. S. A., 109, 11540 (2012).
- Zhou, Y.; Saito, M.; Miyamoto, T.; Novak, P.; Shevchuk, A. I.; Korchev, Y. E.; Fukuma, T.; Takahashi, Y., Nanoscale Imaging of Primary Cilia with Scanning Ion Conductance Microscopy. Chem. 2018, 90 (4), 2891-2895.

