Helical polymers: Functions, synthesis, and chirality
Functionalization of scanning probe with helical polymers to detect cancer related metabolic compounds with scanning probe microscopy

Katsuhiro Maeda, Professor, Nano Life Science Institute (WPI-NanoLSI), Kanazawa University
Helical molecules and chirality
Katsuhiro Maeda is conducting research on the synthesis and applications of dynamic helical polymers. His objective is to develop novel chiral π-conjugated (macro)molecular systems to detect the chirality of target molecules as changes in the absorption, luminescence, visible color, or electrical properties of the molecules.
“Many biological molecules are chiral,” says Maeda. “We can describe molecules in terms of ‘enantiomers’, that is, chiral molecules that are non-superimposable mirror images of one another. Enantiomers of amino acids so-called ‘L’ and DNA are ‘D’. Humans can discriminate between enantiomers —for example, ‘L’-glutamate has umami and ‘D’ does not. In pharmaceuticals, one enantiomer is OK but the other one can cause side effects.”
Functions and synthesis of helical polymer
Some well-known applications of helical polymers include chiral stationary phases that are used to separate enantiomeric compounds; asymmetric catalysts exhibiting high enantio-selectivity; chiral sensors; and circularly polarized luminescence materials for 3D displays and optical communications.
Static, dynamic, and foldamers are the three main types of helical polymers. “I am focusing on dynamic helical polymers in my research,” says Maeda. “The unique features of dynamic helical polymers include chiral amplification, helix-helix transition, and change of helical pitch. We are using these properties for the NanoLSI project.”
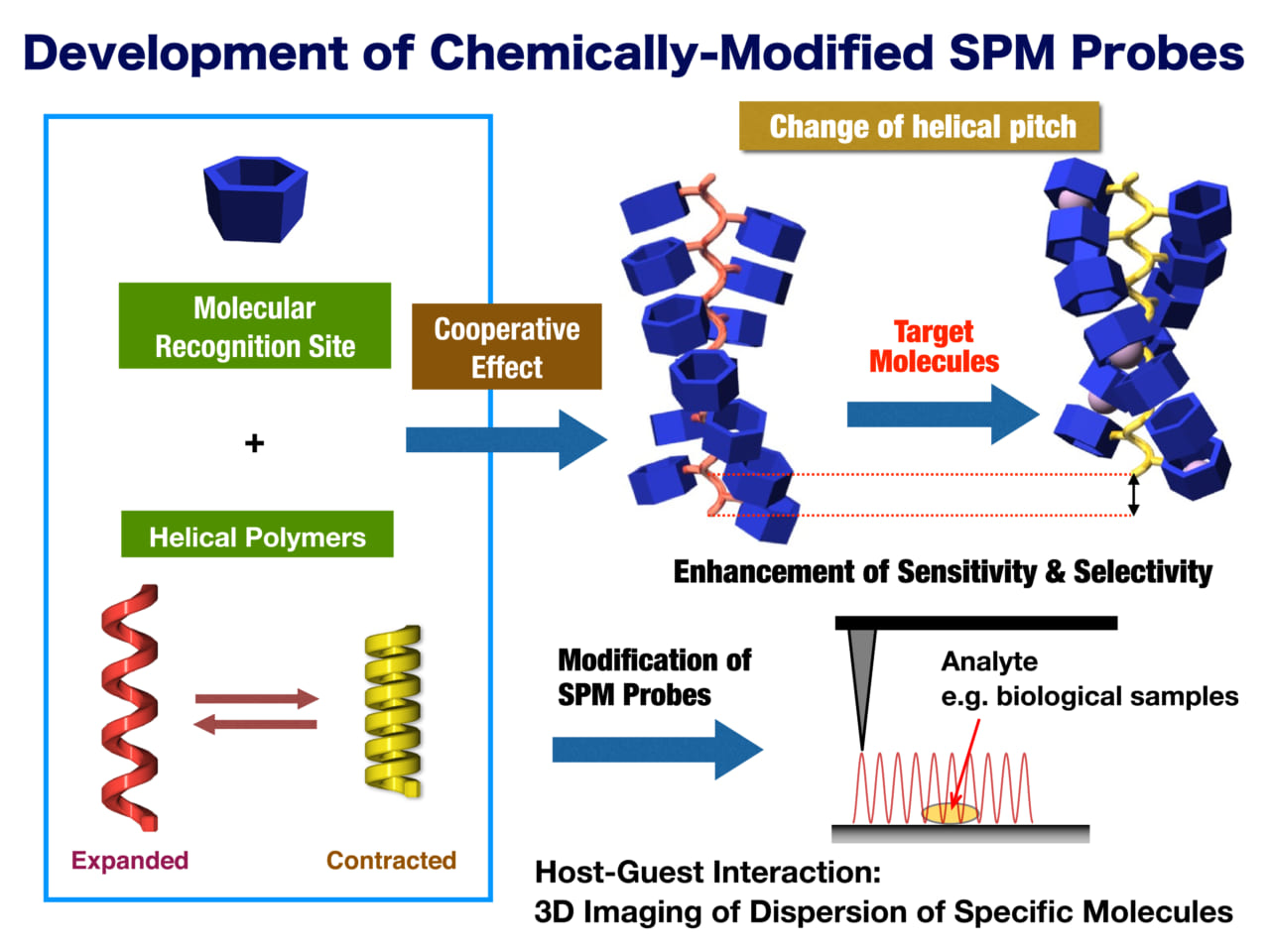
Development of host-modified AFM probes
Maeda is collaborating with researchers at the NanoLSI to detect cancer related metabolic compounds by combining his expertise with scanning ion-conductance microscopy (SICM). The basis for detection exploits the fact that dynamic helical polymers cooperatively change their conformation—such as helical pitch and helical sense—in response to both chiral and achiral external stimuli. Dynamic helical polymers can detect various external stimuli with high sensitivity producing electrical or spectroscopic signals.
“In our approach, we can expect cooperative changes in the pitch of helical polymers through interaction with target molecules,” explains Maeda. “We are modifying SPM probes to investigate host-guest interaction and 3D imaging of the dispersion of specific molecules in biological samples.”
Maeda started his research on helical molecules for his doctorate. “Why do humans have helical molecules is one of many questions that I would like to answer about homo-chirality,” says Maeda. “We are made up of ‘L’-amino-acids and ‘D’-sugars. But, the origin of biological homochirality is still completely unsolved. It’s an intriguing area of research.”
Research highlights
Two recent papers are “Direct Detection of Hardly Detectable Hidden Chirality of Hydrocarbons and Deuterated Isotopomers by a Helical Polyacetylene through Chiral Amplification and Memory”, J. Am. Chem. Soc. [1] and “Switchable Enantioseparation Based on Macromolecular Memory of a Helical Polyacetylene in the Solid State”, Nature Chemistry [2].
References
- Maeda et al., “Direct Detection of Hardly Detectable Hidden Chirality of Hydrocarbons and Deuterated Isotopomers by a Helical Polyacetylene through Chiral Amplification and Memory”, J. Am. Chem. Soc., 140, 3270−3276, (2018). DOI: 10.1021/jacs.7b10981
- K. Shimomura et al., “Switchable Enantioseparation Based on Macromolecular Memory of a Helical Polyacetylene in the Solid State”, Nature Chemistry, 6, 429, (2014). DOI: 10.1038/NCHEM.1916

