Exploring the intracellular space by scanning probe microscopy

Clemens M. Franz, Associate Professor, Nano Life Science Institute (WPI-NanoLSI), Kanazawa University
Visualizing proteins molecules “at work”
“I was born in East Berlin and experienced the fall of the Berlin Wall, which really opened up the world for me,” says Clemens Franz, an associate professor at the WPI-NanoLSI, Kanazawa University. “In terms of my academic background, after an MSc in biochemistry at the Free University Berlin, I moved to University College London where I completed my doctorate in Cancer Cell Biology in 2003. This research led me to think about how the myriad of cells in the human body manage to move and communicate with each other. So, I moved to the Max-Planck-Institute Dresden and later the Karlsruhe Institute for Technology where I developed AFM technics for observing the dynamics of living cells with nanometer resolution. In 2018 I joined the WPI-NanoLSI at Kanazawa University, where I am working with the world’s leading researchers in this area to develop AFM technology to directly observe the dynamics of individual protein molecules in their natural cellular environment.”
Many conventional approaches to visualizing molecules, such as electron microscopy, require sample fixation, thereby preventing the direct observation of the dynamics of individual molecules. One of the most innovative solutions to overcome these limitations is high-speed atomic force microscopy (HS-AFM), which was developed by researchers at Kanazawa University and forms the core technology at WPI-NanoLSI, Kanazawa University. “High-speed AFM enables direct nanoscale observation of the dynamics of individual biomolecules,” says Franz. “It’s amazing how a mechanical tip can yield in-depth information about living cells at work!”
Correlative AFM and light microscopy
Franz’s research at WPI-NanoLSI is based on his expertise on combining AFM instruments with optical microscopes. “During my scientific career I have employed a wide range of correlative AFM/light microscopy platforms,” explains Franz. “Examples include confocal/AFM; super-resolution/AFM; electron microscopy/AFM; traction force microscopy; and total internal reflection fluorescence microscopy/AFM. This correlative approach is a powerful means of collecting complementary structural information of living cells.”
Sequential cell de-roofing for probing the interior of living cells
The mechanical cantilever-probe of an AFM enables nanoscale visualization of the surfaces of living biomolecules, but probing the interior of cells without destroying the natural structural integrity of cells is challenging. “Introducing an AFM nanoprobe into the cell interior to explore organelle ultrastructure therefore requires either partially or fully removing the cell membrane—cell de-roofing,” explains Franz. “I am currently developing cell de-roofing methods that are minimally invasive, retain the native cellular environment and be performed without disturbing the structural integrity of the intracellular organelles under investigation.”
Franz has combined cell de-roofing with high speed AFM to successfully image myosin-driven cytoskeleton contraction on the molecular scale for the first time. Actin stress-fibers—also known as ‘intracellular muscles’—display a sarcomeric organization, but there is only limited understanding about their ultrastructure. “We de-roof cells and then continuously image the exposed actin structures by high-speed AFM during contraction induced by ATP”, explains Franz. “In this way we can directly observe molecular changes during actin cytoskeleton contraction.”
Research plans
Franz will continue to refine his approach of combining HS-AFM with cell de-roofing to probe molecular processes driven by myosin motor action. These experiments are expected to yield insights into the formation of cellular chirality, establishment and maintenance of intracellular tension in geometrically confined spaces, and correlation of stress fiber dynamic ultrastructure with cancer cell invasiveness.
“I am confident that ultimately my research will lead to sub-molecular scale HS-AFM studies on the structure and dynamics of additional functional intracellular components.”
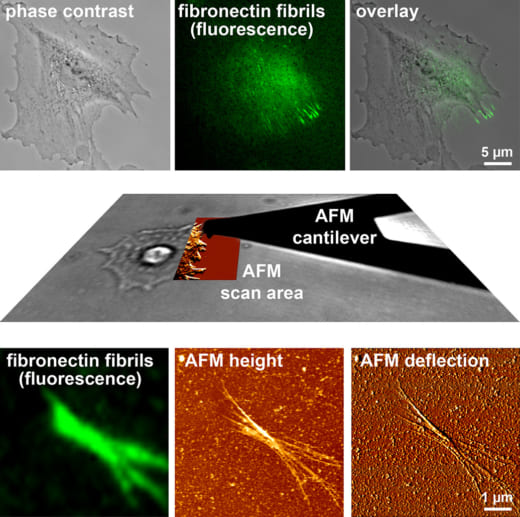
Fig. 1: Combined AFM and fluorescence microscopy to study cell-mediated fibronectin fibrillogenesis (Gudzenko and Franz, MoCB 2015).
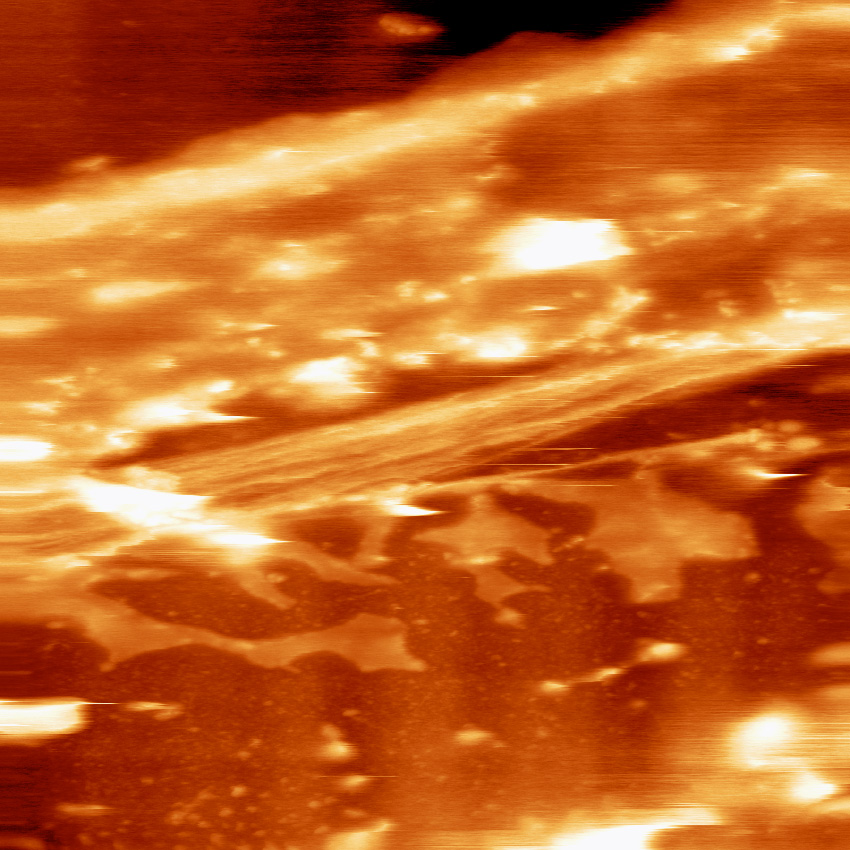
Fig. 2: Exposed actin filaments in a partially de-roofed cell.
References
- C Blaue C, J Kashef, CM Franz*, “Cadherin-11 promotes neural crest cell spreading by reducing intracellular tensionmapping adhesion and mechanics in neural crest explants by atomic force microscopy,” Sem in Cell and Dev Biol 73: 95-106 (2018).
- RP Lange, M Bachmann, SF Becker, G Abbruzzese, D Alfandari, MC Kratzer, CM Franz*, J Kashef “Cadherin-11 localizes to focal adhesions and promotes cell-substrate adhesion,” Nat Comm 7:10909 (2016).

