Cancer biology: Visualizing molecular mechanisms governing stemness and cancer progression
Insights into the importance of metabolic control in maintaining the functions of hematopoietic stem cells and suppressing leukemogenesis

Atsushi Hirao, Professor, Nano Life Sciences Institute (WPI-NanoLSI), Kanazawa University
Blood cells do not live forever. They have a finite lifetime and in healthy humans are being continuously produced by the proliferation of a relatively small number of so-called hematopoietic stem cells (HSCs) found in bone marrow. This cycle of stem cell renewal is critical for healthy tissues, and its disruption can lead to various forms of diseases including bone marrow failure and leukemia.
“Our recent research has shown that metabolic control plays a critical role in maintaining the stem cell properties, so called stemness, and that its dysregulation causes progression of hematopoietic malignancy such as leukemia,” says Atsushi Hirao. “It is becoming apparent that deeper knowledge of molecular mechanisms of metabolic regulation will be important for devising therapeutic strategies for dealing with leukemia stem cells.”
Notably, Hirao and Yuko Tadokoro have discovered that gut microbiota affects stem cell dynamics, so diet and micro-environment are important factors in cancer progression in the case of leukemia [1].
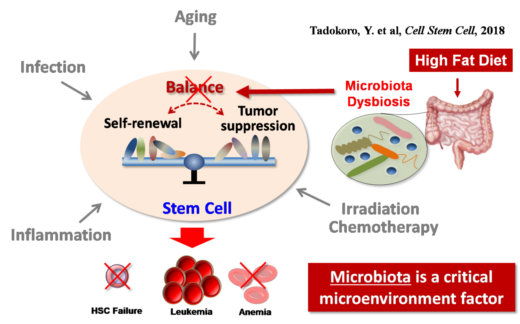
Fig.1 Importance of microenvironments for stem cell regulation
The group led by Hirao is planning to undertake the following projects at the NanoLSI.
Nanoscale analysis of stem cells to clarify how the intracellular ultrastructures change during the differentiation process. This work is being carried with members of RIKEN who have developed advanced nanoscale imaging technologies. “We are developing label-free whole bone marrow cells analysis technology with recognition of HSCs based imaging data using deep learning,” explains Hirao. “We want to analyze changes in the bone marrow micro-environment due to nutritional load, such a fatty diets, and accompanying hematopoietic stem cell abnormalities.”
Another project is characterization of metabolites associated with cancer. Currently there is a lack of technology for real time imaging of metabolites. “We are working with our colleague Tomoki Ogoshi at the NanoLSI to develop chemical sensors for biomarkers using macrocycles,” says Hirao. “Metabolic enzyme as therapeutic targets that govern cancer malignancy will be determined using CRISPR libraries produced by another colleague, Masaya Ueno. The main challenge will be developing materials with high specificity. I hope that our interdisciplinary research will lead to many breakthroughs in cancer biology.”
Research highlights
In 2018 Hirao and colleagues reported that the Spred1 molecule influences hematopoietic stem cell self-renewal [1]. Mouse models showed that under normal conditions, Spred1 acts as a negative regulator, while under diet-induced stress (high fat diet), it protects hematopoietic homeostasis. This study by highlighted the complex relationship between Spred1 and hematopoiesis and establishes a link between the function of the protein and dietary stress.
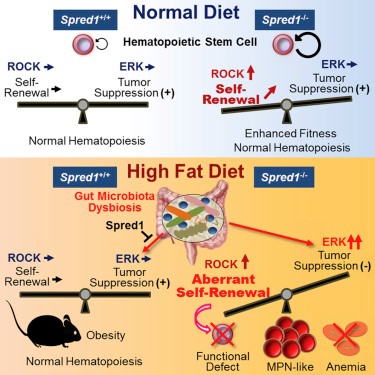
Fig 2: Illustration of the findings in Ref 1.
References
- Yuko Tadokoro etal, “Spred1 Safeguards Hematopoietic Homeostasis against Diet-Induced Systemic Stress”, Cell Stem Cell, 22, 713–725, (2018).
Doi: 10.1016/j.stem.2018.04.002

