Uncovering a Hidden Mechanism in Met Receptor Activation
Researchers at the Nano Life Science Institute (WPI-NanoLSI), Kanazawa University, in collaboration with Osaka University and the National Institutes for Quantum Science and Technology, have uncovered a previously unknown mechanism behind the activation of the Met receptor—a key player in tissue regeneration and cancer progression. Their findings reveal that HGF binding to the membrane-distal domain of Met promotes dimerization at the membrane-proximal domain, which subsequently triggers receptor activation.
Cell-cell communication primarily occurs through the activation of transmembrane receptors such as growth factor and cytokine receptors. Understanding the activation mechanisms of growth factor receptors has been a hot topic in structural biology. Among these, Met, the receptor for hepatocyte growth factor (HGF), plays definitive roles in morphogenesis, tissue regeneration, and cancer metastasis. Met activation is initiated by HGF binding, which induces receptor dimerization. While high-resolution techniques such as X-ray crystallography and cryo-electron microscopy (cryo-EM) have provided valuable insights into this process, they have been limited to truncated forms of Met, leaving the structure of the physiological receptor dimer unresolved. As with other growth factor receptors, elucidating the structure of the full-length receptor dimer in its native cellular environment poses a significant challenge, due to the difficulty of isolating the receptor dimer from living cells and achieving high-resolution structural characterization.
In this study, the physiological Met dimer was chemically cross-linked in living cells prior to isolation. A multifaceted approach was employed to elucidate its structure and investigate the underlying dimerization mechanism. The full-length Met dimer was visualized at the single-protein level using high-speed atomic force microscopy (HS-AFM). To further characterize the HGF-Met complex, the researchers integrated split-luciferase complementary assays, cryo-EM of in vitro reconstituted HGF-Met ectodomain complexes, and molecular dynamics (MD) simulations.
HS-AFM imaging revealed that Met and HGF form a 2:2 complex, with two HGF molecules positioned at the periphery of the dimer. The SP domains of HGF interact with two closely associated Sema domains of Met, a configuration further supported by normal mode flexible fitting. IPT regions of Met are stably connected while exhibiting significant conformational flexibility. The split-luciferase-based detection of ectodomain dimerization and MD simulations demonstrated that this stable connection is mediated by dimerization of the membrane-proximal IPT4 domains of Met. While HGF binding to the Sema domain of Met is known to promote receptor association at the extracellular head region, this study uncovers a critical mechanism of Met activation: HGF not only facilitates head-to-head association but also drives the dimerization of the IPT4 domains, thereby stabilizing the Met dimer.
Beyond elucidating this key activation mechanism of Met receptor, this study also presents a powerful strategy for investigating physiological protein complexes. By combining in-cell cross-linking with HS-AFM, the dynamic structures of such complexes can be visualized at high spatiotemporal resolution. When integrated with computational analysis, this approach offers significant potential for advancing our understanding of complex biological systems, particularly those involving transient or heterogeneous multiprotein assemblies that are challenging to resolve using conventional methods.
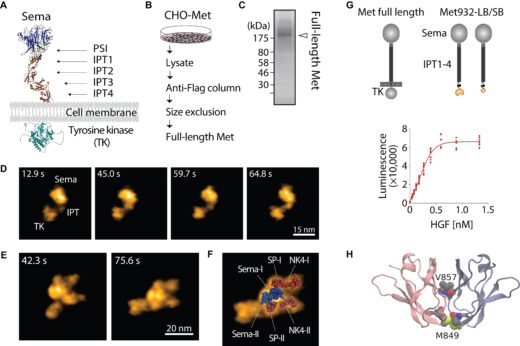
Figure 1. HGF-induced Met dimerization. The AlphaFold3-predicted structure of Met (A). Schematics of full-length Met purification (B). SDS-PAGE and silver staining of purified full-length Met (C). HS-AFM images of full-length Met on mica (D). HS-AFM images of 2:2 HGF-Met complex isolated from living cells (E). Normal mode flexible fitting of the atomistic model of the HGF-Sema structure to the HS-AFM image (F). Split-luciferase complementary assay demonstrating HGF-induced Met dimerization at IPT4 domain (G). Molecular model of an IPT4 dimer (H). Adapted with permission from ACS Nano 2025, 19 (48), 40746–40758. © 2025 American Chemical Society.
Article
- Title
- Ligand Binding to the Membrane-Distal Domain of the Met Receptor Induces Dimerization at the Membrane-Proximal Domain
- Author
- Neval Yilmaz*, Hiroki Tanino, Shun Sakuraba, Romain Amyot, Holger Flechsig, Atsushi Matsumoto, Hidetoshi Kono, Leonardo Puppulin, Mikihiro Shibata, Hiroshi Aoyama, Tsuyoshi Inoue, Kunio Matsumoto*, and Katsuya Sakai*
- Journal
- ACS Nano
- Publication date
- Nov 25, 2025
- DOI
- 10.1021/acsnano.4c17358
- URL
- https://pubs.acs.org/doi/10.1021/acsnano.4c17358

