Scientists develop light-controlled method to trigger brain signals.
Researchers at the Nano Life Science Institute (WPI-NanoLSI), Kanazawa University, report in ACS Nano the successful creation of artificial synaptic vesicles that can be remotely controlled by near-infrared (NIR) light. By embedding a phthalocyanine dye into lipid bilayers, the team achieved local heating that modulates membrane permeability, enabling precise release of neurotransmitters such as acetylcholine. These findings demonstrate that nanoscale heating can control communication between nerve cells.
The work opens new avenues for non-genetic modulation of neuronal activity, with potential applications in neuroscience, drug delivery, and bioengineering.
The team led by Satoshi Arai demonstrated that embedding vanadium phthalocyanine dye (VPc) into liposome membranes allows light-induced local heating confined at the molecular level (Figure1). Unlike conventional temperature-sensitive liposomes, which rely on bulk heating, this approach enables reversible and highly localized release of cargo molecules without causing widespread thermal damage.
Background and Context
Controlling communication between nerve cells is central to understanding how the brain works and to treating neurological disorders. In nature, this communication relies on synaptic vesicles—microscopic sacs that release neurotransmitters such as acetylcholine at just the right time and place. Scientists have long sought artificial systems to mimic this process, which could help repair damaged nerve pathways, develop new treatments for brain and muscle diseases, and create precision drug delivery tools.
Current methods often require genetic modification or heating entire tissues, both of which carry risks of unwanted side effects and damage to delicate biological systems. Optical approaches using near-infrared light are particularly attractive because they can penetrate tissues safely and be focused with high spatial accuracy. The new study addresses this challenge by showing, for the first time, that neurotransmitter release can be controlled through **localized nanoscale heating** of lipid membranes—without disturbing the surrounding environment.
Key Findings
In laboratory experiments, the researchers encapsulated acetylcholine within VPc-liposomes and showed that NIR pulses could trigger rapid neurotransmitter release at defined sites. This release was sufficient to induce calcium flux in muscle cells and neuronal responses in the ‘Drosophila’ brain (Figure2). Molecular dynamics simulations further confirmed that VPc preferentially localizes within lipid membranes, ensuring efficient thermal confinement and effective payload release.
“By confining heat within the lipid bilayer, we achieved precise control of neurotransmitter release in thermally delicate biological systems,” the authors write. “This light-modulated vesicle system represents a promising platform for studying neuronal communication and developing novel therapeutic strategies.”
Potential Impact
The research highlights a new concept of “NIR light-modulated artificial synaptic vesicles”, which could be further extended to targeted drug delivery systems, bio-prosthetics, and micro-scale tools for neuroscience. The approach could also inform the design of future technologies in regenerative medicine, neuromodulation, and bio-inspired nanodevices.
Related figures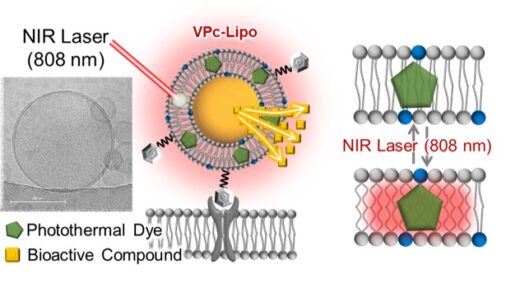
Figure1. A liposome capable of on-demand release of bioactive compounds upon near-infrared (NIR) laser irradiation. A photothermal dye (VPc) is embedded in the lipid membrane, and irradiation with NIR light induces localized heating, which loosens the lipid membrane and enables controlled release. (Figures modified from the published article with permission from the publisher.)
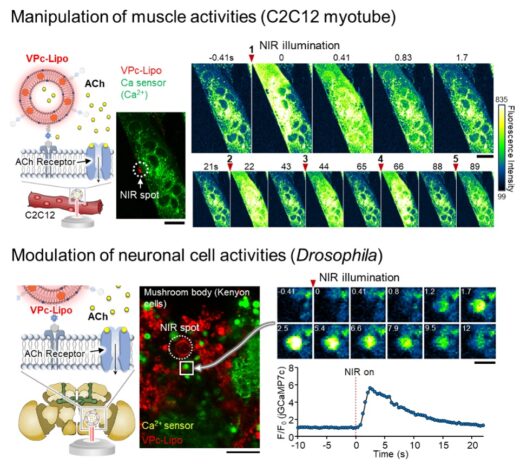
Figure2. Application as an artificial synaptic vesicle. (Upper panel) Schematic illustration of acetylcholine (ACh) release toward differentiated muscle cells and evaluation of acetylcholine receptor activation using calcium imaging. (Lower panel) Schematic illustration of ACh release toward the Drosophila brain (ex vivo) and observation of neuronal activity using calcium imaging. (Figures modified from the published article with permission from the publisher.)
Glossary
- Liposomes– Spherical vesicles made of lipid bilayers, often used to deliver drugs or biological molecules.
- Photothermal effect – Conversion of light energy into heat at the site of absorbing molecules.
- Near-infrared light (NIR) – Light with wavelengths between ~700–1000 nm, which can penetrate biological tissues with minimal damage.
- Artificial synaptic vesicle– An engineered liposome designed to mimic natural vesicles that release neurotransmitters at nerve synapses.
Article
- Title
- Harnessing Dye-induced Local Heating in Lipid Membranes: A Path to Near-Infrared Light-Modulated Artificial Synaptic Vesicles
- Author
- Satya Ranjan Sarker, Takeru Yamazaki, Keitaro Sou, Ichiro Takemura, Yusuke Kurita, Kayoko Nomura, Mari Ichimura, Takahito Suzuki, Ayumi Kai, Takumi Araki, Shinnosuke Hattori, Taniyuki Furuyama, Young-Tae Chang, Taketoshi Kiya, and Satoshi Arai.
- Journal
- ACS Nano
- Publication date
- Jul 27, 2025
- DOI
- 10.1021/acsnano.5c08482
- URL
- https://doi.org/10.1021/acsnano.5c08482

