Protein droplets in the nucleus guard against cancer
Scientists at the Nano Life Science Institute (WPI-NanoLSI), Kanazawa University, have discovered how a gene-regulating protein forms tiny liquid-like droplets inside the cell nucleus (the compartment that stores and manages DNA) to guard against cancer. Their study, published in Nature Communications, shows that these protein droplets act as control centers that keep tumor-suppressor genes switched on.
Guarding the genome
Our genetic material is tightly packed inside the cell nucleus, wrapped around proteins called histones. To keep the right genes active, cells rely on proteins like CHD1, which reorganize this DNA structure when needed.
The Kanazawa team led by Katsuya Sakai, discovered that CHD1 uses a flexible region to form tiny liquid-like droplets, called condensates, that act as hubs for controlling the activity of crucial cancer-guarding genes. These condensates help bring together DNA, RNA, and regulatory proteins to keep tumor-suppressor genes functioning properly.
When protection fails
Normally, CHD1 condensates act as hubs that guard tumor-suppressor genes. But this protective system can break down. A cancer-associated mutation known as E1321fs deletes part of CHD1’s structure that is essential for forming condensates. Without these droplets, CHD1 cannot properly regulate key tumor-suppressor genes, including TP53 and CDKN1B.
As a result, cells carrying this mutation lose an important layer of defense and become more vulnerable to uncontrolled growth and cancer development.
Experimental approach
To investigate this mechanism, the researchers combined state-of-the-art imaging and molecular biology tools:
- High-speed atomic force microscopy (HS-AFM): captured how CHD1 proteins interact with DNA and assemble into condensates at the nanoscale.
- Droplet assays: tested how CHD1 forms condensates in the presence of DNA, RNA, and nucleosomes.
- Confocal microscopy: visualized condensate formation in living cells, comparing normal CHD1 with the mutant form.
- CRISPR-Cas9 genome editing: engineered human cells carrying the E1321fs mutation to track its impact on tumor-suppressor genes.
- Mouse experiments: showed that restoring the missing part of CHD1 reactivated condensate formation and reduced tumor growth.
Together, these experiments revealed how condensate failure leads directly to a breakdown in cancer protection.
A molecular network for cancer protection
The team also mapped how CHD1 droplets interact with other molecules. They found that the condensates attract RNAs and epigenetic regulators such as the MLL complex, another system often disrupted in cancers. This suggests that CHD1 droplets serve as molecular gathering points to coordinate multiple layers of tumor suppression.
Author’s comment
“Our findings reveal a new way in which cells guard themselves against cancer,” says Sakai. “By showing how this protein organizes DNA into droplets that keep tumor-suppressor genes active, we hope to open up new possibilities for cancer therapies that target condensate dynamics.”
Future perspectives
While this study establishes that condensate failure can drive cancer, several questions remain. The team notes that it is still unclear which sequences within CHD1 drive condensation and how condensate size and concentration are regulated inside living cells.
Future research
- Explore the biophysical rules controlling condensate formation in the crowded environment of the nucleus.
- Investigate whether restoring condensate dynamics could provide a new therapeutic strategy for cancers carrying CHD1 mutations.
- Examine how CHD1 works together with other condensate-forming proteins to maintain genome integrity.
Glossary
- Nucleus: The compartment inside a cell where DNA is stored and managed.
- CHD1: A chromatin-remodeling protein that helps control access to DNA by rearranging how it is wrapped around histones.
- Condensates (liquid-like droplets): Tiny, membrane-free compartments inside cells that concentrate proteins and nucleic acids to regulate biological processes.
- Tumor-suppressor genes (e.g., TP53, CDKN1B): Genes that prevent cells from dividing uncontrollably; when disabled, cancer risk increases.
- E1321fs mutation: A cancer-associated mutation that removes part of CHD1, disrupting its ability to form condensates.
- High-speed AFM (HS-AFM): A powerful imaging tool that allows direct visualization of molecules moving and interacting at nanometer resolution.
- CRISPR-Cas9: A gene-editing method used to precisely introduce mutations in cells to study their effects.
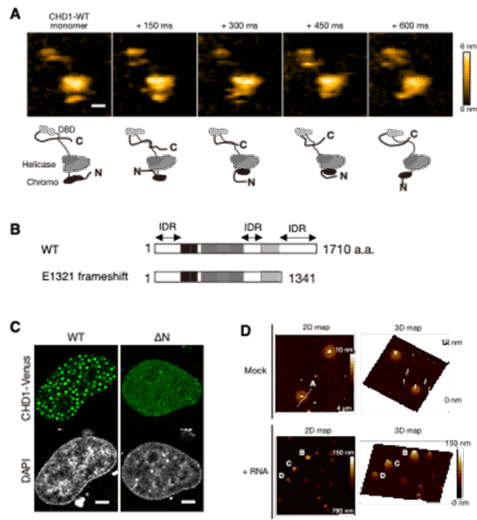
Figure 1. Structural features of CHD1 and visualization of its condensate formation. Single-molecule HS-AFM imaging revealed that CHD1 contains flexible intrinsically disordered regions (IDRs) at its N- and C-termini and in the central region (A). The cancer-associated E1321 frameshift mutation results in the loss of the C-terminal IDR (B). In the nucleus, CHD1 forms liquid–liquid phase–separated condensates via its IDRs (C). HR-AFM imaging of purified CHD1 showed that these condensates adopt soft, disk-like structures (D). Modified from Tsukamoto et al., Nature Communications (2025), CC BY 4.0.
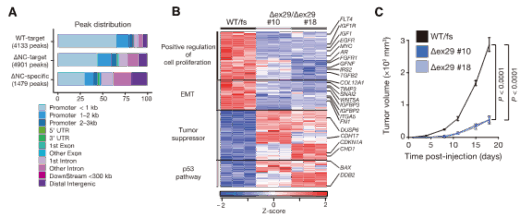
Figure 2. CHD1 condensability is essential for gene regulation and tumor suppression. CHD1’s ability to form condensates is required for its localization to gene promoters (A). In prostate cancer cells carrying the CHD1 E1321fs mutation lacking the C-terminal IDR, tumors grew in nude mice, whereas restoring the deleted region suppressed tumor growth (B) and oncogene expression (C). Modified from Tsukamoto et al., Nature Communications (2025), CC BY 4.0.
Article
- Title
- Condensation-dependent interactome of a chromatin remodeler underlies tumor suppressor activities
- Author
- Yasuhiro Tsukamoto, Atsuki Kawamura, Ayhan Yurtsever, Hidefumi Suzuki, Nichole Marcela Rojas-Chaverra, Hiroki Sato, Daisuke Ino, Takehiko Ichikawa, Weilin Wei, Shojiro Haji, Dominic Chih-Cheng Voon, Akinobu Matsumoto, Kunio Matsumoto, Hidehisa Takahashi, Noriyuki Kodera, Takeshi Fukuma, Yoshihiro Ogawa, Masaaki Nishiyama, and Katsuya Sakai.
- Journal
- Nature Communications
- Publication date
- Oct 30, 2025
- DOI
- 10.1038/s41467-025-64655-w
- URL
- https://www.nature.com/articles/s41467-025-64655-w

