From Molecular Interactions to Material Function: Unraveling Water’s Effect on Chitin Nanocrystals
Researchers at the Nano Life Science Institute (WPI-NanoLSI), Kanazawa University, report in the Journal of the American Chemical Society the use of three-dimensional atomic force microscopy (AFM) and molecular dynamics simulations to determine the structure of water in the hydration of different types of chitin nanocrystals, and how this affects their mechanical properties, reactivities, and interactions with enzymes and reactants.
Chitin is a naturally occurring polymer with a number of attractive mechanical and chemical properties that many are seeking to mimic in bioengineered materials. Naturally occurring chitin has one of two crystal structures known as α – with the molecules aligned antiparallel – and β – with the molecules aligned in parallel. The nanoscale structure of chitin greatly affects the chemical and mechanical properties of the material, and here the structure that water forms around the fibres when they are hydrated can play a significant role. However, until now the details of these different structures were not well understood. Now researchers led by Ayhan Yurtsever and Takeshi Fukuma at Nano Life Science Institute (WPI-NanoLSI), Kanazawa University, together with collaborators Kazuho Daicho, Tsuguyuki Saito, and Noriyuki Isobe from the University of Tokyo, and molecular dynamics experts led by Fabio Priante and Adam S. Foster from Aalto University, Finland, have used 3D AFM and molecular dynamics to study the different structures and how water forms on them when they are hydrated for different pH levels (Fig. 1) The results of this study provide explanations for differences in how the two structures interact with enzymes and reactants.
Atomic force microscopy gauges surface topography and chemical information by monitoring the strength of forces exerted on a nanoscale tip attached to a cantilever. The researchers used a modified AFM known as 3D-AFM, which enabled them not only to image the morphology of chitin nanocrystals but also to investigate the three-dimensional local organization of water molecules surrounding these nano structures. In their report, they noted a high degree of long-range order in the β chitin fibres, whose structure has so far been less thoroughly explored. They describe how the occasional breaks in that order “lead to a structure resembling partially bitten corncobs or a brickwork pattern”. Their AFM imaging also showed how the molecular arrangement runs right through the fibre. “These different structural components are not merely external aggregates;” they explain in the report. “Instead, they constitute an integral part of the chitin fiber(Fig. 2).”
The researchers also investigated the structures under different pHs, to see how this might affect the hydrated architectures of the chitin fibres. They found that the high level of crystallinity observed was preserved in acetic acid buffer solutions pH 3-5. Some of the most significant insights came from studying the water structure and hydrogen bonding on the two crystalline types of chitin (Fig. 3) They showed how the larger grooves in α chitin allowed greater accumulation of water, which formed a hydration barrier for interactions with external ions and molecules making them less reactive. The repulsive forces for hydration were also higher for α chitin. They suggest this may explain why certain enzymes react with chitin in only one crystalline form and not the other. Furthermore, they propose that the lower energetic penalty associated with the structured hydration environment of β-chitin facilitates more rapid enzymatic access and substrate turnover. These insights could inform the development of bioprotonic applications– devices based on the transport of protons as opposed to electronics – and hydrogels since the hydration layer affects ion and molecular diffusion.
“Collectively, this work links nanoscale interfacial structure to rational design strategies, advancing the effective development of sustainable, bio-based nanomaterials for energy and biomedical applications,” they conclude in their report. “Additionally, it provides valuable insights for the computational modeling of chitin surface interactions, crystallosolvate formation, and enzymatic hydrolysis, supporting the development of future material design strategies.”
Glossary
Atomic force microscopy
This imaging technique uses a nanosized tip at the end of a cantilever that is scanned over a sample. It can be used to determine the topography of a sample surface from the change in the strength of forces between the tip and the sample with distance, and the resulting deflection of the cantilever. It was first developed in the 1980s but a number of modifications have augmented the functionality of the technique since. It is better suited to imaging biological samples than the scanning tunnelling microscope that had been previously developed because it does not require a conducting sample.
Chitin
Chitin is a polysaccharide that comprises a substantial component of the shells of marine crustaceans, insect exoskeletons and cell walls in fungi and algae, among other living organisms. Among the attributes that have attracted so much interest in the material are its nontoxicity, antibacterial properties, its tunability in terms of surface structure and chemistry and the nematic crystalline form of hydrated chitin. Potential applications include drug delivery, bone-tissue engineering, sensing, photonic devices, green electronics, self-healing hydrogels, shape-memory bionanocomposites among many others.
The parallel alignment of the fibres in β chitin result in weaker bonds between chitin sheets that enhance the chemical reactivity and allow water to intercalate within the crystal structure. However until this study there were critical gaps in our understanding of β chitin structures, particularly how water intercalates into the structures.
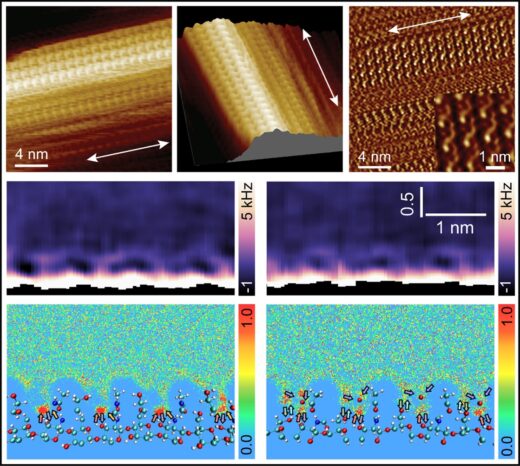
Figure 1. High-resolution AFM images of β-chitin nanocrystal surfaces and the interfacial molecular organization of water near these surfaces.
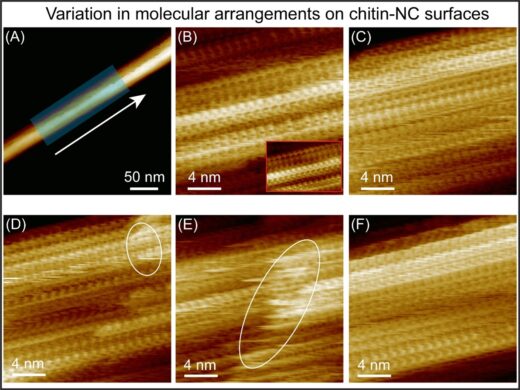
Figure 2: (A) AFM topography image, showing an individual β-chitin NCs on mica, acquired in water. (B–F) High-resolution AFM images recorded along the fiber axis (across the shaded region in (A)), each covering an area of 20–30 nm × 20–30 nm, revealing the structural variations across the crystal surface. Ellipses highlight regions with fluctuating disordered domains on the surface, indicating the boundary between vertically stacked chitin sheets.
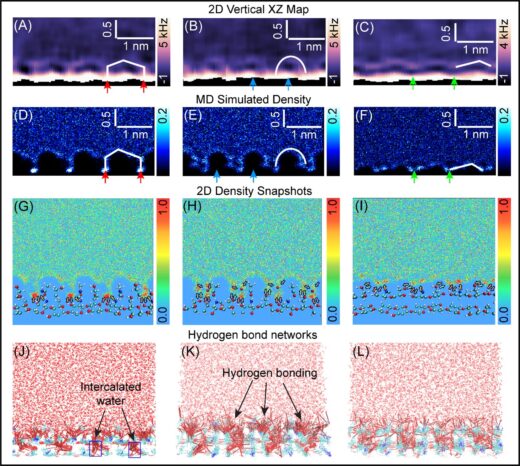
Figure 3: Comparison between water-oxygen density maps and experimentally obtained vertical ∆f maps. (A–C) Vertical 2D-xz ∆f maps taken along the chitin chain direction. (D–F) Simulated vertical 2D water-oxygen density maps along the chain direction at different lateral positions on the (1-20) crystalline plane of the β-chitin NC. The red, blue, and green arrows indicate the identical hydration features between the simulation and experiment. (G–I) Water-oxygen density snapshots (40) with overlaid chitin molecular structures. (J–L) Hydrogen-bonding networks formed between water molecules and the underlying chitin substrate.
The copyright for all figures in this article is attributed as follows:
© 2025 American Chemical Society.
Article
- Title
- Interplay between β-Chitin Nanocrystal Supramolecular Architecture and Water Structuring: Insights from Three-Dimensional Atomic force Microscopy Measurements and Molecular Dynamics Simulations
- Author
- Ayhan Yurtsever, Kazuho Daicho, Fabio Priante, Keisuke Miyazawa, Mohammad Shahidul Alam, Kazuki Miyata, Akinori Yabuki, Noriyuki Isobe, Tsuguyuki Saito, Adam S. Foster and Takeshi Fukuma
- Journal
- Journal of the American Chemical Society
- Publication date
- Oct 16, 2025
- DOI
- 10.1021/jacs.5c08484
- URL
- https://doi.org/10.1021/jacs.5c08484

