HS-AFM accelerates the research and development (R&D) of novel vaccines and therapeutics for combating pore-forming toxins
Summary
Researchers from Kanazawa University and Vietnam National University have published their latest findings in Archives of Biochemistry and Biophysics: “Imaging oligomers of alpha-toxin (Hla) variants using high-speed AFM and neutralizing Hla hemolytic activity with antisera”. Led by Dr. Kien Xuan Ngo (Biochemistry, HS-AFM), Dr. Ngan Thi Phuong Le (Biochemistry/Biology, HS-AFM), and Dr. Hoang Duc Nguyen (Biochemistry/Biology), this study highlights the significant role of HS-AFM—pioneered by Toshio Ando, Noriyuki Kodera, and Takayuki Uchihashi—in advancing vaccine and therapeutic research and development (R&D) against bacterial infections caused by pore-forming toxins (PFTs).
Staphylococcus aureus is a major antibiotic-resistant pathogen, causing infections such as bloodstream infections, abscesses, pneumonia, and endocarditis. Its invasion into host cells relies on MSCRAMMs and alpha-toxin (Hla), which oligomerizes into transmembrane pores, leading to cell lysis. Hla is highly antigenic, conserved in clinical strains, and crucial for S. aureus virulence. Studies indicate that strains lacking Hla show reduced invasiveness, and patients with high anti-Hla antibody levels exhibit protection against S. aureus sepsis.
Hla toxoids, including HlaH35A, HlaH35L, and HlaH35LH48L, lack hemolytic activity and serve as promising antigens for vaccine development. This study used HS-AFM to visualize these variants on POPC/Chol membranes, revealing that His35 mutations allowed oligomer formation, whereas HlaH35LH48L remained monomeric. The His35-His48 region was identified as critical for oligomerization. Hemolytic activity of HlaWT was significantly reduced in the presence of these toxoids at a 1:5 ratio. Additionally, mice immunized with these toxoids developed anti-Hla antibodies capable of neutralizing HlaWT toxicity on RBCs. These findings support the potential of Hla variants in vaccine and therapeutic strategies against S. aureus infections.
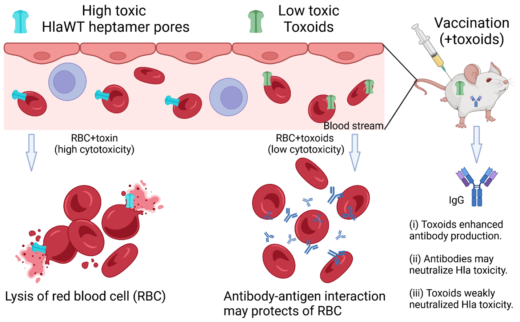
A summary of the immune-stimulated effects of Hla toxoids/variants on antibody production in mice and their neutralization to prevent the cytotoxicity of HlaWT on rabbit RBCs. © 2025, Le, Ngo, Nguyen et al. Archives of Biochemistry and Biophysics.
Perspectives and future challenges
Their findings demonstrate that Hla variants can bind to the surface of POPC/Chol liposomes, suggesting that liposomes can serve as a biological trap to neutralize this toxin. Indeed, sphingomyelin liposomes with a high cholesterol content (66 mol/%) have been shown to efficiently sequester the potent Hla toxin and significantly reduce tissue necrosis in a murine abscess model. This opens up a promising approach for the treatment of S. aureus infections, particularly those caused by antibiotic-resistant strain. Using liposomes to sequester HlaWT may help reduce cytotoxic effects on host cells, minimize tissue damage, and support the immune system in clearing the infection more effectively. These results provide a scientific basis for the research and development of liposome-based therapies as a potential strategy for combating S. aureus infections in the future.
Article
- Title
- Imaging oligomers of alpha-toxin (Hla) variants using high-speed AFM and neutralizing Hla hemolytic activity with antisera
- Author
- Ngan Thi Phuong Le, Kien Xuan Ngo*, Trinh Thi Ngoc Nguyen, Linh-Thuoc Tran, Hoang Duc Nguyen* ̶ *Corresponding authors.
- Journal
- Archives of Biochemistry and Biophysics
- Publication date
- Mar 25, 2025
- DOI
- 10.1016/j.abb.2025.110403
- URL
- https://www.sciencedirect.com/science/article/pii/S000398612500116X

