Frequency modulated AFM sheds light on how dipeptides help organize, immobilize and catalyze
Researchers at Nano Life Science Institute (WPI-NanoLSI), Kanazawa University, observe the configuration of different dipeptides on graphite electrodes and the subsequent arrangement of catalytic hemin on them to get an idea of the factors affecting its catalytic activity.
Self-assembled peptides have shown great promise for immobilizing and exploiting enzymes in catalytic applications. However, so far little has been known as to the structures of these self-assembled peptides and how this might affect the function of the enzyme immobilized. Now researchers led by Ayhan Yurtsever and Takeshi Fukuma at Kanazawa University, WPI-NanoLSI and Marie Sugiyama and Yuhei Hayamizu at Institute of Science Tokyo have compared the morphology and activity of hemin adsorbed on different dipeptide nanostructures using atomic force microscopy (AFM), cyclic voltammetry and H2O2 reduction reactions to see which offers the best performance and why (Figure 1).
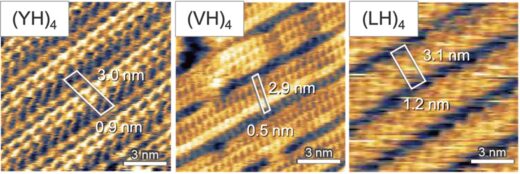
The researchers used frequency modulated atomic force microscopy to study the structures that self-assembled from droplets of (XH)4 peptide solution on a graphite electrode, where H is histidine and X is an amino acid – either Y, L or V. Their observations indicated that dipeptides self-assemble into repeating nanostructures resembling 2D crystals, with (YH)4 exhibiting the most ordered and stable configuration (Figure 2).
They then replaced the droplet of peptide solution with a droplet of hemin solution and used AFM to observe the configuration of the hemin as it bound to the peptide structure. They found the hemin aggregated on the dipeptide structures, and further observations with high-speed AFM revealed that the hemin formed wires as well as aggregates, and that while the wires were stationary the aggregates seemed to hop along and between rows of the dipeptide (Figure 3).

The findings from this study were published online in the American scientific journal ACS Nano on April 15, 2025 (US Eastern Time), and were featured on the journal’s front cover.
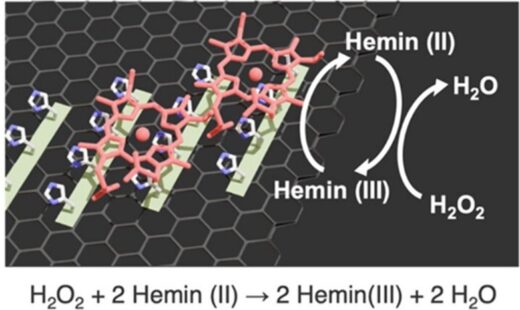
The researchers used cyclic voltammetry to measure how densely hemin bound to the dipeptide structures and found that it bound most densely to (YH)4. They attribute this to the tyrosine in (YH)4, which interacts with porphyrin through π-π interactions. However, adding just porphyrin to the dipeptide bound hemin structures had little effect, from which they deduced that “the Fe atom in hemin is critical for its interaction with peptides, and that the binding is not solely driven by π−π stacking interactions,” as they report in ACS Nano. While the density of hemin binding to (LH)4 was close to that for (VH)4, they found it bound slightly more densely to (VH)4, which they attribute to the greater hydrophobicity.
On applying a reduction current to the electrodes, the iron in hemin is reduced to the ferrous (+2) oxidation state. It can then reduce H2O2, thereby recovering its ferric oxidation state. Comparisons of how quickly the hemin bound structures reduce H2O2 revealed that hemin bound to (YH)4 had the highest catalytic activity, although this is unlikely due to the greater density of hemin at this surface since the densities for all three dipeptides were all within the same order of magnitude. Instead, the researchers suggest the greater reducing power of hemin bound to (YH)4 is on account of the more stable scaffold offered by that dipeptide (Figure 4).
“This research highlights the potential of simple peptide designs to create artificial enzymes with robust and durable catalytic interfaces for electrochemical applications,” conclude the researchers in their report. “Furthermore, the peptides’ ability to self-assemble on two-dimensional materials makes them promising candidates for biosensing applications.”
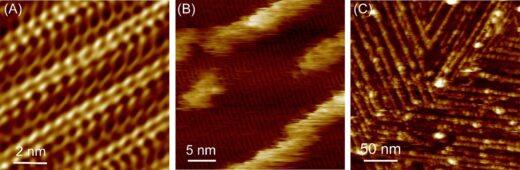
Peptide self-assembly and subsequent hemin adsorption on graphite substrate. (A) High-resolution AFM image showing the molecular arrangement of (YH)4 peptides, forming 2D crystalline lattices on graphite in water. (B) Initial stage of hemin binding on self-assembled (YH)4 peptide nanostructures, revealing the formation of relatively unstable molecular rows along peptide lattices. (C) At later stages of adsorption, the hemin molecules form more stable and densely packed rows that ultimately cover the underlying peptide lattices completely.
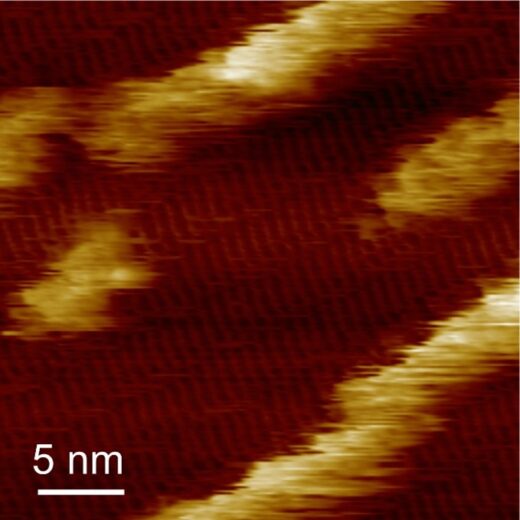
Fig 3: In situ AFM image showing the immobilization of hemin on self-assembled (YH)4 peptides, revealing the formation of hemin molecular rows along peptide lattices.
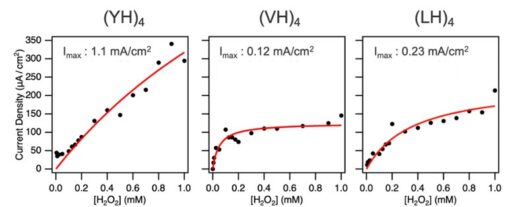
Fig. 4: Current density at −0.8 V as a function of H2O2 concentration for each peptide, with fitting curves shown as red solid lines. Imax represent the maximal current density.
Copyright for all figures ©2025 American Chemical Society
Glossary
Redox chemistry describes a host of reactions that involve the gaining (reduction) and losing (oxidation) of electrons by ions in the reaction. It sometimes manifests as the gaining of hydrogen (reduction) or oxygen (oxidation). The generation of water and oxygen from H2O2 is an example of a redox reaction where H2O2 is reduced to H2O and O2.
Article
- Title
- Hierarchical Assembly of Hemin-Peptide Catalytic Systems on Graphite Surfaces
- Author
- Marie Sugiyama, Ayhan Yurtsever, Nina Uenodan, Yuta Nabae, Takeshi Fukuma, and Yuhei Hayamizu
- Journal
- ACS Nano
- Publication date
- Feb 16, 2025
- DOI
- 10.1021/acsnano.4c15373
- URL
- https://pubs.acs.org/doi/10.1021/acsnano.4c15373


In the 2000s Toshio Ando at Kanazawa University was able to improve the scanning speed to such an extent that moving images could be captured. This allowed people to use the technique to visualize molecular processes for the first time.