Atomic force microscopy upgrade captures 3D images of calcite dissolving
Researchers at Nano Life Science Institute (WPI-NanoLSI), Kanazawa University, implement modifications to their high-speed atomic force microscopy that simultaneously improve resolution and speed, while enabling direct measurements of 3D structures to provide conclusive evidence of a contested hydration layer forming as calcite dissolves.
Understanding the dissolution processes of minerals can provide key insights into geochemical processes. Attempts to explain some of the observations during the dissolution of calcite (CaCO3) have led to the hypothesis that a hydration layer forms, although this has been contested. Hydration layers are also important as they play a role in a number of processes including adhesion, corrosion and wetting, as well as the folding, stability and recognition of proteins. Now researchers led by Kazuki Miyata, Adam S. Foster and Takeshi Fukuma at the Nano Life Science Institute (WPI-Nano LSI) at Kanazawa University in Japan have successfully upgraded their atomic force microscope to retrieve imaging data with the time and spatial resolution needed to obtain 3D structure images that provide direct evidence of a hydration layer forming during the dissolution of calcite.
The hypothesis of a hydration layer forming during the dissolution of calcite was prompted by simulations of the process, which pointed to the production of a Ca(OH)2 layer across “transition regions” as calcite dissolves. Despite being unstable in the bulk or on flat terraces, Ca(OH)2 can appropriate some stability from step edge structures, although the mechanism behind this is not well understood. This could explain the stability of the Ca(OH)2 next to the step edges but since the transition regions observed in experiments span several nanometres, the authors had posited the possibility that the Ca(OH)2 acquires its stability through indirect interactions with the step by means of a hydration structure. However, as the researchers point out in their report, hydration effects remain “poorly understood” as techniques for imaging changes in solid–liquid interfacial structures are lacking.
Atomic force microscopy (AFM) obtains high resolution images by using a nanoscale cantilever to feel the surface a little like the needle of a record player feels out the grooves in vinyl. However, despite a huge step change in the rate of image acquisition that can be achieved with the invention of high-speed (HS) AFM, AFM has still suffered a little from a trade-off between speed and spatial resolution. Efforts to apply it to study dissolution processes is also hampered because the tool is designed to scan the topologies and interactions across 2D surfaces, and dissolution of minerals involves 3D structural changes.
Previous work had expedited the higher resolution “frequency modulated” (FM) AFM so that the image acquisition time was reduced from a minute to just 0.5 s/frame. This upgrade allowed the authors to image the transition region from which they inferred the presence of a hydration layer, but some extrapolation was required to extract 3D structure information from comparison of the 2D-AFM data to 3D simulation, leaving some to doubt the conclusions. Modifications of AFM to extract 3D force data using AFM have previously been demonstrated, although once again despite some improvements to speed things up, to around 1 minute/frame the image acquisition time remained prohibitive for observing dynamic processes.
The authors get around all these drawbacks by combining the HS-FM-AFM with 3D-SFM. This involved increasing the bandwidth of their 3D-SFM while maintaining a force resolution of 10-100nN, fast synchronization of the signals in the lateral scanning and 3rd dimension, and fast recording of the cantilever frequency shifts. With these in place the researchers were able to capture 3D-SFM images in just 1.6 s/frame. They used the approach to image the dissolution of calcite.
“The HS-3D-SFM images produced in the present work clearly show the 3D distribution predicted by the simulations, thus supporting the existence of an extended hydration layer,” they point out in their report.
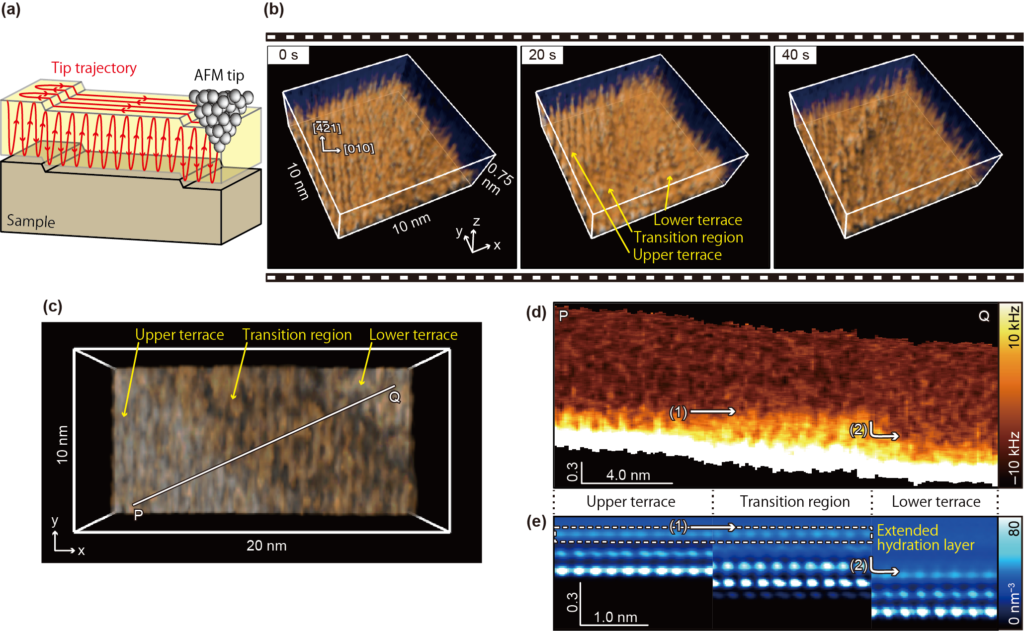
Fig. 1: High-speed 3D-SFM imaging
(a) Imaging principle of 3D-SFM, where the distribution in three-dimensional space can be measured by scanning the AFM tip horizontally and vertically. The grey spheres represent individual atoms constituting the tip. (b) 3D-SFM images obtained near the calcite step edge during its dissolution at 5 s / 3D image. The water distribution on the terraces and transition region is obtained, with clearly different structures in them. (c) Another example of a 3D-SFM image obtained at 1.6 s/3D image. (d) Vertical cross-sectional image obtained over the terrace and transition region. The thickness of the respective hydration structures (dark orange areas) can be seen. The top edge of the respective hydration structure in the upper terrace and transition region is approximately the same height (arrow (1)), while the position of this top edge is significantly lower in the lower region (arrow (2)). This suggests that the extended hydration layer is formed at arrow (1). (e) Classical molecular dynamics simulation. This indicates that the water in the upper terrace and transition region is at the same height, consistent with the results in (d).
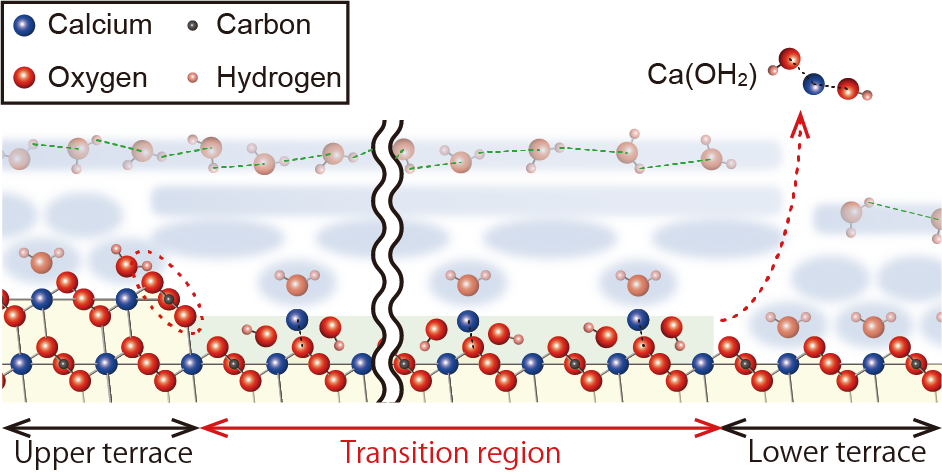
Fig.2 A model of the formation and stabilisation mechanism of the transition region.
Waters in the hydration layer above the upper terrace and transition zone are hydrogen bonded to each other and form a network. This extended hydrogen bonding network would be energetically favored, and Ca(OH)2 does not desorb from the surface because it is inhibited by this network. However, the energetic favorability of this extended layer would decrease as the distance from the step edge increases. Finally, Ca(OH) 2 desorbs at a point where its energy to desorb exceeds that of the Ca(OH)2. Finally, Ca(OH)2 desorbs at a point where the energy at which it attempts to desorb exceeds the energy of network stabilisation.
© 2024 Miyata et al., Nano Letters
Glossary
AFM
Atomic force microscopy was first established in the mid 1980s by Gerd Binnig, Calvin Quate and Christophe Gerber. It uses a nanoscale cantilever with a nanoscale tip attached to it, such that interactions between the tip and the surface result in a deflection of the tip. Thanks to the atomically sharp end of the tip sub nanometre resolution can be achieved.
Frequency modulated AFM
The first demonstration of AFM achieved a lateral resolution of 30 Å in air. A number of modifications to the initial set up helped to improve the device. By avoiding contact with the sample, the approach could be used to study delicate and soft materials. Tapping mode or frequency modulated AFM helped to improve the sensitivity of the device for certain set ups. Here the end of the cantilever flaps up and down and the change in frequency due to the interactions with the surface is monitored.
High-speed AFM
In 2008 Toshio Ando at Kanazawa University demonstrated how modifications that improve the feedback bandwidth and phase detection as well as optimizations to the cantilever could speed up the image acquisition rate sufficiently to allow AFM movies of dynamic processes.
AFM is better suited to imaging biological samples than the scanning tunnelling microscope that had been developed before it because it does not require a conducting sample. The development of HS-AFM allowed people to use the technique to visualise molecular processes for the first time.
Later developments helped people to speed up FM-AFM to capture images at a rate of 0.5 s/frame too. The NanoLSI researchers used this approach to study the dissolution of calcite previously but they could only monitor the 2D structure change. To draw conclusions on the 3D shape they assumed the presence of the Ca(OH)2, calculated the 3D structure with the Ca(OH)2 layer and a hydration layer on top, infer the forces from that, and hence the frequency changes an AFM would measure and the resulting 2D height map, and finally compare that with the image obtained from 2D-FM-AFM.
3D-AFM
Further developments of 3D-AFM made it capable of monitoring the forces acting on the tip in all three dimensions. Although originally quite slow because of the complexity of the tip motions involved, the NanoLSI researchers found they could speed it up by modulating the vertical height of the tip sinusoidally while scanning laterally.
This “3D scanning force microscopy (3D-SFM)” allowed images to be captured at a rate of 1 minute/frame – fast enough to avoid image distortion due to tip drift but not yet fast enough to monitor dynamic processes, which was enabled by combining 3D-AFM with HS-FM-AFM. This allowed direct monitoring of dynamic 3D structure changes.
Article
- Title
- High-speed three-dimensional scanning force microscopy visualization of subnanoscale hydration structures on dissolving calcite step edges
- Author
- Kazuki Miyata, Kosuke Adachi, Naoyuki Miyashita, Keisuke Miyazawa, Adam S. Foster, Takeshi Fukuma
- Journal
- Nano Letters
- Publication date
- Aug 26, 2024
- DOI
- 10.1021/acs.nanolett.4c02368
- URL
- https://doi.org/10.1021/acs.nanolett.4c02368

