Endoscopy of a living cell on the nanoscale
Researchers at Kanazawa University report in Science Advances a new technique for visualizing the inside of a biological cell. The method is an extension of atomic force microscopy and offers the promise of studying nanoscale inner cell dynamics at high resolution in a non-destructive way.
In order to advance our understanding of how biological cells function, visualizing the dynamics of intra-cellular components on the nanoscale is of key importance. Current techniques for imaging such dynamics are not optimal — for example, fluorescence microscopy can visualize ‘labeled’ molecules but not the target components themselves. A label-free, non-destructive method has now been developed by Takeshi Fukuma from Kanazawa University and colleagues: nanoendoscopy-AFM, a version of atomic-force microscopy that can be deployed within a living cell. The research was carried out as a collaboration between Kanazawa University and the National Institute of Advanced Industrial Science and Technology (AIST), with Marcos Penedo, the lead author of the publication reporting the new method, recently moving from Kanazawa University’s Nano Life Science Institute (WPI-NanoLSI) to the École Polytechnique Fédérale de Lausanne, Switzerland.
The principle of AFM is to have a very small tip move over the surface of a sample. During this ‘xy’ scanning motion, the tip, attached to a small cantilever, will follow the sample’s height (‘z’) profile, producing a measurable force on the cantilever. The magnitude of the force can be back-converted into a height value; the resulting height map provides structural information about the sample’s surface.
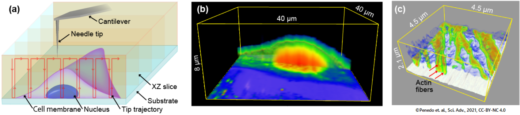
The researchers designed a novel AFM setup where the needle-like tip is brought in and out of the interior of a cell. The process is reminiscent of an endoscopy — the procedure of looking at an organ from the inside, by inserting a small camera attached to a thin tube into the body — which is why Fukuma and colleagues call their technique nanoendoscopy-AFM. Letting the nanoneedle travel an ‘xyz’ trajectory going in and out of the cell results in a 3D map of its structure. They tested the technique on a cell from the so-called HeLa cell line commonly used in medical research. In a scanned volume of 10 x 10 x 6 µm3, internal granular structures could be clearly identified.
During a scan, the nanoneedle penetrates the cell membrane (and the nuclear membrane) many times. The scientists checked whether this repeated penetration does not cause damage to the cell. They performed a viability test on HeLa cells by using two fluorescent marker molecules. One molecule emits green fluorescence from a living cell, the other red fluorescence from (the nucleus of) a dead cell. The researchers found that when using nanoprobes smaller than 200 nm, nanoendoscopy-AFM does not lead to severe damage to cells.
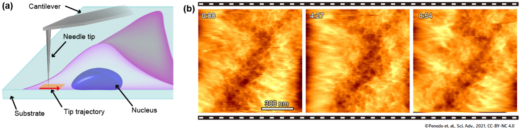
The method is also particularly useful for probing surfaces within the cell, for example the inner side of the cell membrane or the surface of the cell nucleus. Fukuma and colleagues call this application 2D nanoendoscopy-AFM, and point out that it could be combined with high-speed AFM resulting in a powerful technique for studying the nano-dynamics of the interior of living cells in physiological environments.
The scientists stress that AFM is the only method that allows label-free imaging of biomolecular systems, and conclude that their technique will enable the “direct observation, analysis and manipulation of intracellular and cell surface dynamics to gain insights about the inner cell biological processes … increasing the ability to understand biological phenomena.”
Image
Fig.1 Principle and example of intra-cellular 3D imaging by nanoendoscopy AFM. (a) Principle. (b) 3D-AFM image of the live HeLa cell. (c) 3D-AFM image of the actin filaments in the live fibroblast cell.
Fig.2 Principle and example of intra-cellular 2D imaging by nanoendoscopy AFM. (a) Principle. (b) Successive 2D-AFM images of the mesh-like structure consisting of actin filaments at the inner surface of the live fibroblast cell.
(Penedo et. al., Sci Adv. 2021, CC-BY-NC 4.0)
Background
Atomic force microscopy
Atomic force microscopy (AFM) is an imaging technique in which the image is formed by scanning a surface with a very small tip attached to a small cantilever. Horizontal scanning motion of the tip is controlled via piezoelectric elements; the vertical position of the tip changes as it follows the sample’s height profile, generating a force on the cantilever that can be measured and back-converted into a measure of the height. The result is a height map of the sample’s surface. As the technique does not involve lenses, its resolution is not restricted by the so-called diffraction limit as in optical microscope, for example.
Fukuma and colleagues have now extended the principle of AFM for studying the interior of living cells, by inserting the tip–probe in a needle-like way through a cell’s membrane into the cell.
Researchers’ information
Article
- Title
- Visualizing intra-cellular nanostructures of living cells by nanoendoscopy-AFM, Science Advances 7, eabj4990 (2021) .
- Author
- Marcos Penedo, Keisuke Miyazawa, Naoko Okano, Hirotoshi Furusho, Takehiko Ichikawa, Mohammad Shahidul Alam, Kazuki Miyata, Chikashi Nakamura, Takeshi Fukuma
- Journal
- Science Advances
- Publication date
- Dec 22, 2021
- DOI
- 10.1126/sciadv.abj4990
- URL
- https://www.science.org/doi/10.1126/sciadv.abj4990