New Study Explains How Inflammation Causes Gastric Cancer
Abstract
Researchers from Kanazawa University and the Japan Agency for Medical Research and Development have solved the decades-old mystery of how stomach bacterium Helicobacter pylori causes gastric cancer. Using mouse models and human cancer cell lines, they showed that inflammation resulting from bacterial infection leads to the proliferation of gastric epithelial cells, which ultimately form gastric tumors. By blocking the protein pathway responsible for this proliferation, they prevented gastric tumor formation.
Kanazawa, Japan – In 1982, researchers reported a link between chronic gastritis and stomach bacterium Helicobacter pylori, triggering a flurry of research into this newly-identified pathogen. These studies made it clear that in addition to its involvement in gastritis, H. pylori was a significant factor in the development of both peptic ulcers and gastric cancer. But while the link between the bacterium and disease was clear-cut, exactly how H. pylori caused gastric tumors remained the subject of much debate.
Now, almost four decades later, researchers from Kanazawa University and the Japan Agency for Medical Research and Development have finally shown how inflammation caused by H. pylori infection causes the proliferation of gastric epithelial stem cells, leading to gastric tumors.
In a report published recently in cancer genetics journal Oncogene, the researchers describe how they built on previous findings to solve the mystery.
“We previously showed that tumor necrosis factor alpha (TNF-α), a cytokine that causes inflammation, promotes gastric tumor formation by activating a protein called NOXO1,” says lead author of the study Dr Kanae Echizen. “What we didn’t know was exactly how NOXO1 induces tumor formation in the stomach.”
NOXO1 is a component of the NOX1 complex, which produces tissue-damaging molecules called reactive oxygen species (ROS). ROS, or more accurately, the oxidative stress caused by these molecules, can result in mutations in the DNA of stomach cells, leading to tumor formation. Inflammation caused by H. pylori infection also produces ROS, increasing oxidative stress in the stomach.
In the newly-published study, the research team showed that inflammation caused excess production of NOX1-complex proteins in response to signals from NF-κB, a regulatory protein that turns on genes to combat stress or bacterial infection, and which is a major player in the inflammatory response. However, most importantly, they found that NOX1/ROS signaling caused gastric epithelial stem cells to multiply uncontrollably, resulting in tumor formation.
Knowing this, the researchers used a drug to suppress the activity of the NOX1 complex, which immediately halted the growth of gastric cancer cells. Even more excitingly, disruption of Noxo1 in a mouse gastritis model stopped the proliferation of epithelial stem cells.
“We have finally been able to show that inflammation enhances the expression of NOXO1, which induces the proliferation of gastric epithelial stem cells, leading to gastric tumors,” explains Dr Masanobu Oshima, senior author of the study. “Gastric cancer is the fourth most common cancer worldwide and has the second highest mortality rate. If we can disrupt the NOX1/ROS signaling pathway in situ, we may be able to prevent the development of this aggressive disease.”

Figure 1.
A schematic illustration of the role of Noxo1 and NOX1 in the inflammation-associated gastric tumorigenesis. Helicobacter pylori infection causes chronic gastritis, which further induces expression of Noxo1 through TNF-α/NF-κB inflammatory cytokine pathway. Induction of Noxo1 as well as other NOX1 complex, such as NOX1, Noxa1 and Cyba, leads to activation of NOX1 complex and ROS production. Increased ROS level in the inflamed gastric mucosa results in expansion of SOX2-positive stem cell population, which induces gastritis-associated gastric metaplasia and hyperplasia. This Noxo1-involved inflammatory pathway contributes to gastric tumorigenesis in the H. pylori-infected stomach.

Figure 2.
ROS production in the normal stomach (left), gastritis tissue (center), and gastric tumors (right) of mouse models. ROS was detected by DHE staining (top) and nuclei of stomach cells are stained with DAPI (bottom). Note that ROS level is increased in proliferative zone of gastritis (yellow dashed lines) and pan-gastric tumor cells.
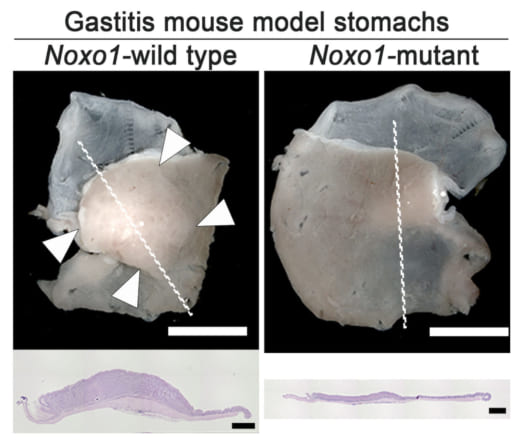
Figure 3.
Representative photographs of gastritis-associated gastric mucosal hyperplasia (arrowheads, left) found in the gastritis mouse model, which was significantly suppressed by mutation of Noxo1 gene (right). Histology sections of the lined mucosal areas are shown in the bottom (H&E staining), indicating complete suppression of gastritis-associated hyperplasia by Noxo1 disruption.
Article
- Title
- NF-κB-induced NOX1 activation promotes gastric tumorigenesis through the expansion of SOX2-positive epithelial cells
- Author
- Kanae Echizen, Keigo Horiuchi, Yayoi Aoki, Yoichi Yamada, Toshinari Minamoto, Hiroko Oshima & Masanobu Oshima
- Journal
- Oncogene
- Publication date
- Jan 30, 2019
- DOI
- 10.1038/s41388-019-0702-0
- URL
- https://www.nature.com/articles/s41388-019-0702-0

